What is Herceptin?
Herceptin is the trade name for the anti-cancer drug trastuzumab. Trastuzumab is a monoclonal antibody that selectively
targets the extra-cellular domain of the human epidermal growth factor receptor 2 protein (HER2).1 An
over expression of HER2 is seen in approximately 15%–20% of invasive breast cancers.2 This
causes an increase of HER2 protein on the surface of the tumour cells, activating the HER2 receptor.1 Women
with tumours that over-express HER2 have been found to have a decreased likelihood of recurrence-free survival and overall
survival, compared to women with HER2 negative breast cancer.3 Trastuzumab inhibits the
proliferation of tumour cells that over-express HER2. The exact mechanism by which it does this is unclear. It appears
that trastuzumab attaches itself to the HER2 protein, contributes to apoptosis (cell death), reduces HER2 expression,
alters various cellular cycles and potentiates the effects of chemotherapy. Trastuzumab may also have extracellular effects,
e.g, mediating immune recognition.2
What is Herceptin indicated for?
Herceptin is indicated for the treatment of women with metastatic breast cancer who have tumours that over-express HER2.
It is used alone for patients who have already received one or more chemotherapy regimens or in combination with taxanes
(e.g. paclitaxel) for those who have not received chemotherapy.
Herceptin can also be used for the treatment of HER2 positive early breast cancer in women who have a normal Left Ventricular
Ejection Fraction (LVEF) following surgery (lumpectomy or mastectomy) and chemotherapy.
What is the treatment regimen for Herceptin?
Herceptin is administered by intravenous infusion once a week. New evidence has shown that a three-weekly dosing schedule
is also effective.4
There are currently three regimens that have been investigated for adjuvant treatment of HER2 positive early breast
cancer;
- Herceptin for 12 months after chemotherapy (anthracycline +/- taxane)
- Herceptin for 12 months in combination with chemotherapy (taxane), and after anthracycline
- Herceptin for 9–10 weeks in combination with chemotherapy (taxane), and before anthracycline
How effective is Herceptin?
Advanced breast cancer
The use of Herceptin in metastatic breast cancer is generally accepted by most health professionals. Its effectiveness
was investigated in a randomised controlled trial (n=469) which compared standard chemotherapy with and without trastuzumab.
It was found that the addition of trastuzumab to the chemotherapy regimen resulted in a longer time to disease progression
(7.4 vs. 4.6 months), a longer duration of response (9.1 vs. 6.1 months) a lower death rate at one year (22% vs. 33%)
and longer survival (25.1 vs. 20.3 months) than chemotherapy alone. Cardiac toxicity was observed in 27% of patients receiving
trastuzumab.5
In a case series (n=222) investigating the use of Herceptin as monotherapy, 4% of women experienced a complete tumour
response to treatment and 12% experienced a partial tumour response i.e. trastuzumab demonstrated anti-tumour effects.
The median duration of survival was 13 months for all women with HER2 positive metastatic breast cancer and 16 months
in a subgroup with HER2 at 3+ levels.6
Age does not appear to be a factor in the effectiveness of Herceptin treatment.4
Early stage breast cancer
Much of the evidence for the use of Herceptin in early breast cancer has been gathered from five major clinical trials.
These trials have shown that Herceptin reduces the risk of recurrence in women with HER2 positive early breast cancer.
Table 1 summarises the characteristics and results of the major trials.
Table 1: Summary of clinical trials investigating the use of Herceptin in early HER2 positive
breast cancer.11,15
| |
|
HERA (Europe)
|
N9831 (arm C)
and NSABP B31
joint analysis (USA)
|
BCIRG 006 (USA) |
FinHer
(Finland) |
| Trial size (n) |
|
3401 |
3351 |
2148 |
231 |
Median follow-up
years |
|
2 |
2 |
3 |
3 |
Trastuzumab
schedule |
|
12 months, after anthracyclines +/- taxanes |
12 months, concurrent with anthracyclines and taxanes |
12 months, concurrent with anthracyclines and taxanes, +/- carboplatin |
9 weeks, concurrent with anthracyclines +/- taxanes |
| Recurrence or death from any cause (%) |
Trastuzumab |
218 (12.8%) |
133 (8.0%) |
128 (11.9%) |
12 (10.4%) |
| |
Control |
321 (18.9%) |
261 (15.5%) |
192 (17.9%) |
27 (23.3%) |
| |
Hazard ratio (95% CI) |
0.64 (0.54–0.76) |
0.48 (0.39–0.59) |
0.61 (0.48–0.78) |
0.42 (0.21–0.83) |
All-cause mortality (%)
|
Trastuzumab |
59 (3.5%) |
62 (3.7%) |
49 (4.6%) |
6 (5.2%) |
| |
Control |
90 (5.3%) |
92 (5.5%) |
80 (7.5%) |
14 (12.1%) |
| |
Hazard ratio (95% CI) |
0.66 (0.47–0.91) |
0.67 (0.48–0.93) |
NR |
0.41 (0.16–1.08) |
| Serious, life threatening or fatal cardiac events (%) |
Trastuzumab |
10 (0.6%) |
51 (3.1%) |
based on 2 year data 25 (2.3%) |
0 |
| |
Control |
1 (0.1%) |
5 (0.3%) |
10 (1.0%) |
0 |
| |
Relative risk (95% CI) |
9.97 (1.28–77.80) |
10.38 (4.15–25.91) |
2.46 (1.19–5.09) |
|
| Number Needed to Treat (NNT) |
Calculated NNT (mortality) |
55.6* |
55.6 |
34.5 |
14.5 |
| |
Calculated NNT
(Disease free survival) |
16.4 |
13.3 |
16.7 |
7.8 |
| * A NNT of 55, for example, means that one extra woman will be alive for every 55 treated for the period
over which the NNT was calculated, in this case two years. |
The Herceptin Adjuvant (HERA) Trial is one of several large trials which tested the efficacy of trastuzumab
administered over a 12 month period. Results of analysis after two years follow up showed that trastuzumab, given once
every three weeks after chemotherapy, achieved a significant improvement in disease-free survival compared with women
treated with chemotherapy alone.7 The joint analysis of two other trials (N9831 and B-31)
showed a significant improvement in disease-free survival with trastuzumab administered concurrently with paclitaxel,
every one or three weeks after chemotherapy, compared with the same chemotherapy schedule alone. Analysis of the BCIRG
trial has also shown a disease-free survival benefit when trastuzumab is administered with docetaxel after chemotherapy,
or with docetaxel and carboplatin.7
Because of the improved disease-free survival with concurrent treatment in the joint analysis of the B31 and
N9831 trials,8 an unplanned interim analysis of the N9831 trial was
undertaken to assist the management of patients receiving sequential treatment in the trial.9 The
analysis compared the results of patients in the sequential Herceptin treatment arm with those in standard care (no Herceptin)
and concurrent treatment arms. The results of this analysis (Table 2) showed that a Herceptin treatment regimen received
concurrently with paclitaxel for 12 weeks, followed by an additional 40 weeks of Herceptin treatment provided greater
disease free and overall survival benefit than a sequential regimen of 12 weeks of paclitaxel, followed by 52 weeks of
Herceptin.9
Table 2: Concurrent vs Sequential Treatment: Unplanned interim analysis of the N9831 trial,
comparing Arm B (sequential) and Arm C (concurrent).9
Pairwise comparison
|
Disease Free
Survival
|
|
Overall Survival |
|
| |
Number of events* |
Hazard Ratio (95% CI) |
Number of events (%) |
Hazard Ratio (95% CI) |
| Concurrent vs. control** |
140 (54 vs. 90) |
0.55 (CI not reported) |
(not reported for N9831 alone) |
(not reported for N9831 alone) |
| Sequential vs. control |
220 (103 vs. 117) |
0.87 (0.67 – 1.13) |
79 |
0.85 (0.55 – 1.33) |
| Concurrent vs. sequential |
137 (53 vs. 84) |
0.64 (0.46 – 0.91) |
56 |
0.74 (0.43 – 1.26) |
Notes
* Numbers of events differ between comparisons because of differences between times of analysis and censoring
of some patients’ results.
** The results for concurrent treatment vs. control are for trials B31 and N9831 jointly and do not differentiate
between the two trials; hence separate results are not available for N9831.
|
Control = AC 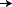 T (12
weeks) T (12
weeks)
Concurrent = AC 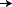 T + H (12 weeks) T + H (12 weeks)
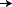 H
(40 weeks) H
(40 weeks)
Sequential = AC 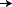 T (12 weeks) T (12 weeks)
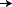 H
(52 weeks) H
(52 weeks) |
| AC = Anthracycline T = Taxane (Paclitaxel) H
= Herceptin |
The FinHer Trial involved an alternative treatment sequencing and duration to the commonly used 12-month period. Results
from FinHer show that trastuzumab administered concurrently with a taxane, over a nine week treatment period, before standard
chemotherapy (including anthracycline) was effective in increasing the recurrence-free survival rate among women with
HER2 positive early breast cancer. No significant cardiac toxicity was observed with this shorter treatment period and
this group had fewer decreases in cardiac function (LVEF) than the control group.10 In addition
the FinHer study had longer follow-up than other studies.
However this study only included 116 women treated with Herceptin and it is uncertain whether a similar result for adverse
cardiac effects would be seen in a larger group of patients, especially as a wider range of pre-existing cardiac disease
was excluded from the study, compared to the other trials.
Although a larger scale trial is needed the small size of the FinHer study indicates an appreciable effect, where disease-free
survival was statistically significant, despite the small number of patients.11
| The evidence of effectiveness of using Herceptin in early breast cancer
has so far centred on disease-free survival. Long-term survival has yet to be adequately assessed due to the number
of follow-up years since the major trials commenced. Caution is warranted in applying the results of these trials to
the whole population. There may be some variability in baseline risk of recurrence of breast cancer depending on the
nature of the chemotherapy regimen used prior to treatment with Herceptin. In addition long term risks of cardiac toxicity
have yet to be evaluated. In some groups of women the side effects associated with trastuzumab may outweigh the benefit
of treatment.12 |
What are the adverse effects associated with Herceptin?
Approximately 50% of patients can expect to experience an adverse reaction to treatment. Most often these are relatively
minor infusion-related effects such as fever or chills. There have been infrequent reports of serious adverse reactions
including dyspnoea, hypotension, wheezing, bronchospasm, tachycardia, reduced oxygen saturation and respiratory distress.
Most adverse reactions can be treated with supportive therapy such as oxygen, beta-agonists and corticosteroids. In rare
cases adverse effects can result in death.4
The most significant adverse effect associated with Herceptin is cardiac toxicity. After one year sequential trastuzumab
therapy in the HERA study 0.6% of patients had severe congestive heart failure (CHF) and 2% had symptomatic CHF. In comparison
0.1% of control patients had symptomatic CHF and none had severe CHF. In addition 3% of patients who received Herceptin
and 0.5% of controls had a significant decrease in LVEF. Seventy-two patients (4%) that were receiving Herceptin withdrew
from the study due to cardiac problems.7 After a median follow up of two years in the HERA
study, it has been calculated that trastuzumab will raise the absolute risk of symptomatic CHF by 2% and by 5% including
sub-clinical harms.
For symptomatic CHF the number needed to harm (NNH) is 51 and the NNH for the risk of all cardiac harms is 20.
This compares with the NNT of 56.7
In the joint analysis of B-31 and N9831, the three year cumulative incidence of severe cardiac events (severe (grade
3/4) heart failure or cardiac death) was 4.1% and 2.9% (respectively) for patients receiving concurrent Herceptin, and
0.8% and 0% for controls. Overall results for the combined trials were not reported. In study B31 34% of the patients
in the trastuzumab arm had a significant decrease in LVEF (≥10% decline to below 55%) versus 17% in the control group.
13 Two
cardiac deaths occurred during these trials.14 In the BCIRG study after 3-year’s median
follow-up 1.9% of patients that received concurrent Herceptin and 0.4% of controls developed CHF (grade 3/4). A LVEF decline
(>10%) was seen in 10% of controls and 18% of concurrent Herceptin patients, however no cardiac related deaths occurred.
15 None
of the patients in the FinHer trial suffered clinically significant cardiac events or cardiac related death.10
What issues still need to be resolved?
There is much evidence in support of the use of Herceptin in both metastatic and early HER2-positive breast cancer.
However, the following issues require further investigation:
- The optimal scheduling of trastuzumab treatment with different chemotherapy regimens, concurrent with taxanes, pre/post
anthracyclines or sequential treatment post chemotherapy
- The comparative clinical effectiveness of 12 months vs shorter treatment periods including assessment of adverse events
- Long-term efficacy follow up and assessment of toxicity
- The risk of recurrence in specific subgroups (e.g. tumours with nodal involvement and with or without hormone receptors)