In this article 
View / Download
pdf version of this article
GENERAL PRACTICES AROUND NEW ZEALAND are increasingly providing intravenous (IV) administration of medicines in the
community, in order to offer treatment to patients who would otherwise need to be admitted to hospital. Different funding
and support structures exist around the country to enable practices to offer these services and as yet there is no national
model for providing community IV treatment. The purpose of this article is to highlight the conditions for which IV treatment
in the community would be appropriate, and the reasons that practices may wish to offer these services, alone or as part
of a treatment network.
The advantages of community-based treatment
In general, given the option, people prefer to receive community-based treatment than be admitted to hospital.1–3 Treating
people in their own communities allows them to remain with family and continue work, education or fulfil other commitments.
In comparison, hospital stays are disruptive and require patients, and their families, to adapt to hospital routines while
experiencing a reduction in privacy and comfort.
The benefits of a community-centric approach extend beyond patient satisfaction. Patients treated at home are not at
risk of acquiring nosocomial (hospital acquired) infections.4 A study of patients with cellulitis, conducted
in Christchurch, showed that community-based treatment resulted in patients receiving a comparable standard of care, with
no significant difference in condition advancement, length of treatment or adverse events, when compared to hospital admission.2 Community-based
treatments also, generally, result in significant cost savings when compared to hospital admission. In one New Zealand
study it was found that 31% of all hospital admissions could have been avoidable.5 Reducing avoidable hospital
admissions can increase the quality of care for patients admitted into secondary care.6 Some patients could
also be discharged from hospital earlier if strategies exist for effective delivery of community-based treatments. As
DHBs attempt to improve service and reduce cost through innovation, it is likely that community-based treatment will become
increasingly common in New Zealand.
IV administration of medicines
Each year many patients are admitted to secondary care for the treatment of acute conditions such as dehydration or
infection, requiring IV administration of fluids or medicines. Primary healthcare professionals are well placed to offer
services for administering IV treatment, given the necessary resources, appropriate patient selection and the provision
of refresher training, if required. In countries such as Australia, Canada, the United States and the United Kingdom,
home based delivery of IV antibiotics has been shown to be safe and cost effective.7–9 Several localised studies,
conducted in the Christchurch and Auckland regions, have also found primary care to be an effective means of delivering
IV treatments to suitable patients.9,10
Deciding who to treat
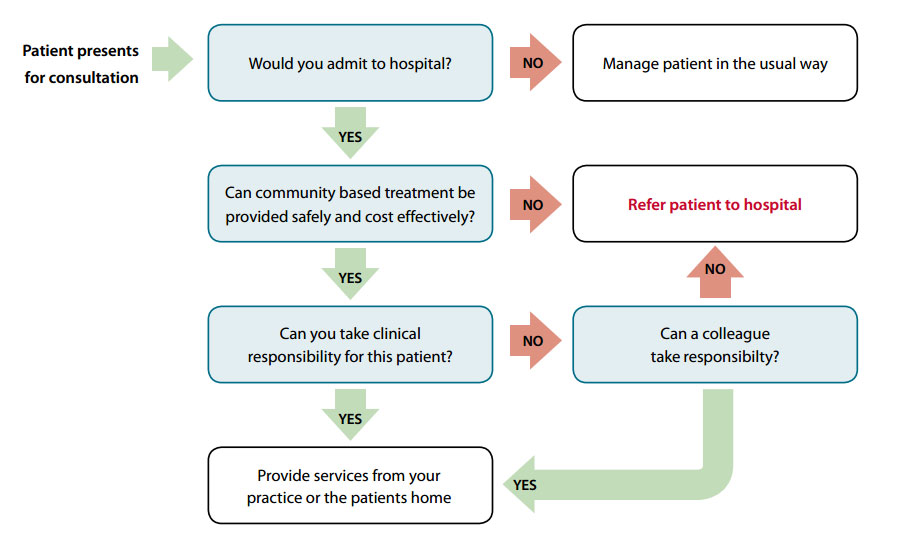
The decision to treat a patient in the practice, or at their home, is based on clinical judgement. An algorithm can
be used to assist this decision (Figure 1 below).
Issues that need to be considered when deciding to administer IV treatment include:
- Does your practice have the equipment, space, time and skills?
- Is the patient old enough? Patients aged under 15 years may present added complications, such as specialised dosing
requirements, and in most circumstances should be referred to secondary care for IV treatment
- Will the infusion(s) be delivered at home or in the clinic?
- Is the medicine stable and suitable to be delivered in a primary care setting?
- Can the required medicines be easily obtained?
- What volume of infusion is required and how long will each treatment last?
- How frequently will the treatment be required?
- Will consultation with a community or hospital pharmacist be of assistance?
Figure 1: Referral process algorithm – adapted from POAC information manual11
What conditions can be treated?
There are no national guidelines on which conditions are most suitable for community-based IV treatment. Primary Options
for Acute Care (POAC) is an organisation that funds practices in the Auckland, Waitemata and Counties Manukau DHBs to
deliver community-based IV services. The most common conditions, for which IV treatments were delivered by these practices,
between June 2010 and June 2011 were:
- Cellulitis – 5388 cases
- Respiratory infection – 2379 cases
- Dehydration – 1036 cases
- Kidney infection – 424 cases
 Individual reports for Auckland, Waitemata and Counties Manukau DHBs are
available from: www.primaryoptions.co.nz/page/News_and_Reports
Individual reports for Auckland, Waitemata and Counties Manukau DHBs are
available from: www.primaryoptions.co.nz/page/News_and_Reports
Cellulitis
Patients presenting with cellulitis represent the largest group suitable for community-based treatment. A United Kingdom
based study found that one-third of patients presenting to hospital with cellulitis could have been treated at home.2 An
analysis of the POAC service in the Auckland region, found that the average cost of treating a patient with cellulitis
in the community was $246.36, compared to nearly $3000 for a hospital admission, which lasted on average 4.4 days.10
The first-line treatment for cellulitis is oral antibiotics.12 Patients presenting with severe cellulitis,
or those that do not respond to oral antibiotics, can be considered for community based IV antibiotic treatment. Recommended
medicines for community based IV treatment of cellulitis may differ from those used in a hospital setting. For example,
POAC recommends treatment with cephazolin (2 g daily) plus oral probenecid (500 mg twice daily),13 as this
is more practical than six hourly administration of flucloxacillin.
N.B. POAC supplies its practices with a “cellulitis kit”. Cephazolin is not funded on the Pharmaceutical
Schedule for this indication.
Admission to hospital is recommended for patients with cellulitis who present with:
- Haemodynamic instability – tachycardia, relative hypotension, severe dehydration or compromised circulation
- Severe pain or swelling
- Unstable risk factors such as heart failure or diabetes
- Severe or worsening symptoms following an animal or human bite
- Periorbital or facial cellulitis (unless very mild)
- Veins that are difficult to cannulate due to age or previous IV drug use
Once the decision to give community based IV antibiotics is made, patients should be reviewed daily and switched to
oral delivery of antibiotics as soon as it is clinically reasonable (usually within 48 hours). The patient should be admitted
to hospital if any of the following are observed:
- No improvement in condition after 24 hours
- Worsening infection and skin necrosis
- Worsening fever and/or pain
- Rising white blood cell count
- Diarrhoea suggestive of Clostridium difficile
Dehydration management
Vomiting and diarrhoea are the most common causes of dehydration, due to excessive fluid loss or reduced fluid intake.
Patients, who present with moderate to severe signs and symptoms of dehydration, may be suitable for community-based IV
treatment, depending on clinical assessment and individual patient factors.
Mild – The patient may have no symptoms other than a mild thirst and concentrated urine. An oral electrolyte
solution can be used for rehydration. In most cases patients will be able to safely manage oral rehydration in their own
home. Depending on the circumstance, it may be advisable to discuss the situation with the patient’s family, as
dehydration can be an indication that a patient is having difficulty coping at home.
Moderate – The patient will have a significant thirst, low urine production, sunken eyes, dry mucous
membranes and may be weak, light-headed and experiencing postural hypotension. Depending on the circumstances, consider
testing glucose and electrolyte levels or taking urine or faecal samples. Rehydration with a specialised oral electrolyte
solution is recommended (e.g. pedialyte or enerlyte).14 The high osmolality and low sodium content of fruit
juices and carbonated drinks may increase gastrointestinal fluid loss.
Alternatively, IV fluid (normal saline – 0.9% NaCl) can be administered. An adult may be given 1000 mL of fluid initially,
and then reviewed. Another 500 – 1000 mL can be given every two to four hours as required. However, no more than 2 L should
be given. The patient should be encouraged to take oral fluids and their status reviewed daily. Patients requiring more
than 2 L of IV saline, due to ongoing losses, should be considered too unstable for treatment in a community setting.13
Severe – This is a serious condition in which patients will often display significant thirst, tachycardia,
low pulse volume, cool extremities, reduced skin turgor, significant hypotension and confusion. Immediate referral to
hospital is recommended.
N.B. Patients, who are experiencing vomiting, may benefit from the administration of an antiemetic such as metoclopramide
at the same time as hydration treatment.14 Metoclopramide is generally not recommended for use in children
and adolescents.
Refer the following patients to secondary care:
- Patients requiring IV fluids for dehydration who meet any of the following criteria should be referred to hospital:13
- Children
- People with diabetes
- Renal failure
- Heart failure
- Septicaemia
- Undiagnosed abdominal pain
- Intracranial symptoms
Caution is also advised for elderly people or people with; pre-existing heart failure, difficulty managing at home,
prolonged symptoms, an evolving illness or recently returned from overseas.
Other situations where community-based IV infusion might be considered include:
- Antibiotic treatment of pyelonephritis
- Antibiotic treatment of respiratory infections
- Chemotherapy in rural settings
Infection control
IV treatment procedures can sometimes result in serious infections. It is important that standard infection control
procedures are followed at each stage of the process.
Hand washing
Hands should be washed with soap and water immediately before and after patient contact. Paper hand towels, and not
hot air dryers, should be used to dry hands. Wrist and hand jewellery should be removed and any cuts or abrasions covered
with a waterproof dressing. Finger nails should be short and clean.
Personal protective equipment
Powder-free gloves should be worn when performing infusion procedures and disposed of in medical waste bags.
Reconstitution
Whenever possible, infusions in a ready-to-use form should be purchased. Health professionals performing reconstitution
need to be aware of the compatibility and stability of solutions and record all calculations and ensure all containers
are well labelled. Aseptic technique should be followed, including disinfecting the tops of all vials, ampoules and bags
with a chlorhexidine and alcohol based solution. Manufactures guidelines should be followed at all times and careful attention
paid to the expiration date of any products used.
 Notes on injectable drugs (6th edition), published by the New Zealand
Hospital Pharmacists’ Association, provides reconstitution instructions for all commonly used medicines in New Zealand.
This resource can be ordered in hard copy or electronic form, from: www.nzhpa.org.nz/media/3240/noidsflyer6.pdf
Notes on injectable drugs (6th edition), published by the New Zealand
Hospital Pharmacists’ Association, provides reconstitution instructions for all commonly used medicines in New Zealand.
This resource can be ordered in hard copy or electronic form, from: www.nzhpa.org.nz/media/3240/noidsflyer6.pdf
Community pharmacists can also assist with information on reconstitution of medicines.
Sterilising reusable equipment
Protocols for cleaning and sterilising should be developed for each practice. All equipment, dressings and solutions
that come into contact with the patient must be sterile. Equipment such as drip stands need to be cleaned regularly. Medicines
and solutions should be stored and used according to the manufacturer’s instructions.
Site selection and placement of the cannula for IV treatment
Before insertion, it is important to confirm the vein will accommodate the gauge and length of the cannula required.
When selecting a cannula, the smallest gauge and shortest length practical should be chosen. The insertion site should
be decontaminated with an antimicrobial solution (such as 2% chlorhexidine and alcohol solution) applied with a sterile
applicator and then allowed to dry.15
In most cases in a primary care setting, the cannula will be removed after each treatment and the patient will be re-cannulated
the next day. However exceptions to this may include patients for whom cannulation was difficult and patients who are “needle
phobic”.
Considerations for site selection and placement of the cannula are detailed in Table 1.
Table 1: Considerations for site selection and placement of the cannula
| Do consider a patient’s19 |
Take care19 |
If possible19 |
- Age
- Condition
- Diagnosis
- Vascular function
- Infusion history
- Treatment frequency, duration and type
|
- Not to use veins in the lower limbs of adults – due to the risk of embolism and thrombophlebitis
- Not to cannulate the feet of people who have diabetes
- To avoid areas of flexion if possible
|
- Select distal portions of upper limbs
- Subsequent insertions should occur in a distal fashion
- Remove hair with clippers or scissors as razors can cause micro-abrasions which may become infected
|
Stablising cannulae left in place
In cases where the cannula is left in situ, it is important that any efforts to stabilise it do not restrict access
to it, or impede its function. Sterile dressings should be applied to the area following insertion. Dressings must be
inspected at regular intervals and changed if they are lifting or blood stained. The insertion site should be clinically
inspected daily for; tenderness, fever without an obvious source, symptoms of local or systemic infection or the presence
of discharge from the cannula insertion site.15 The condition of the insertion site and the integrity of the
device should be briefly documented in the patient notes at each inspection.
Maintenance
Daily flushing of the device with 3 – 5 mL of 0.9% NaCl ensures that it does not become blocked. Further flushing should
occur between the introduction of medicines which do not mix. If any resistance is felt then the cannula should be removed
and reinserted elsewhere. Cannulae should not be left in place for longer than 96 hours.15,16
Removal
If the cannula is being removed due to infection, the tip should be sent for microbiological testing and blood cultures
taken. The tip of the needle should also be examined to ensure it is intact. Any faulty devices should be reported to
the manufacturer.
 The Royal College of Nursing has published standards for infusion therapy,
available from: www.rcn.org.uk (key words: infusion therapy).
The Royal College of Nursing has published standards for infusion therapy,
available from: www.rcn.org.uk (key words: infusion therapy).
Training
IV administration can be associated with a number of adverse events. It is important that clinicians who perform this
technique have adequate training and are familiar and competent with the:
- Anatomy and physiology of the circulatory system
- Reconstitution and mixing of IV medicines
- Various access devices that are available
- Potential problems of vein selection caused by age, inflammation, thrombosis, disease and infection
- Practice of risk management to reduce needle stick injuries and blood spills
- Monitoring and care of the infusion site
- Practice of infection control
- Recognition and management of anaphylaxis
 Further information relating to training for IV skills can be found on
the intravenous nursing website, available from: www.ivnnz.co.nz
Further information relating to training for IV skills can be found on
the intravenous nursing website, available from: www.ivnnz.co.nz
Will this work in your practice?
The implementation of a programme for IV administration of medicines in a general practice can present some challenges.
Issues to consider include; training, equipment supply, timely access to medicines, funding for services and patient selection
criteria. Support services can provide guidance and practical answers to these problems. In some cases, practices may
create their own solutions such as; requesting local community pharmacies stock antibiotics or cellulitis kits, collaborating
and/or diversifying to provide coverage over larger areas, or collective purchasing of equipment to be shared between
practices as required.
Initiatives for primary care IV administration will vary depending on local DHB protocols. Therefore, it is not possible
to outline a “one size fits all” mechanism for their operation or to detail how cost recovery will work. However,
through prior consideration of the concept, primary care is better placed to influence its implementation.
Oesteoporosis and Paget’s disease
Since September 2010, the bisphosphonate zoledronic acid (Aclasta) has been funded under Special Authority for the
treatment of oesteoporosis and Paget’s disease. The medicine may be prescribed and administered in a General Practice.
Zoledronic acid is given once a year, as a slow IV infusion delivered over a period greater than 15 minutes. Patients
must sit, or stand, upright for 30 minutes after taking bisphosphonates orally, therefore zoledronic acid is a useful
alternative for people unable, or unwillingly, to do this. IV infusion can be delivered by a trained Practice Nurse in
any clinic with space available for 30 minutes. IV infusion can also be considered for people likely to be non-compliant
with oral treatment or people who are intolerant to oral bisphosphonates due to gastrointestinal problems.
As there have been reports of renal impairment associated with zoledronic acid, it is important that it is not given
to patients with a creatinine clearance below 35 mL/min. Patients should also be sufficiently hydrated before, and after
the infusion, particularly if they are taking diuretics, or any other medicines that impact on renal function.17
Zoledronic acid is contraindicated in patients with hypocalcaemia and it is recommended that serum calcium levels be
assessed if the patient has; vitamin D deficiency, recently undergone thyroid or parathyroid surgery or has calcium malabsorption.18 Patients
with Paget’s disease of the bone need adequate calcium and vitamin D and may benefit from calcium supplementation
for two weeks following infusion.
In the first few days following treatment, some patients may complain of flu-like symptoms. These symptoms usually
resolve within a day or two and may be alleviated by taking paracetamol with 500 mL of water following the infusion.
This has the added benefit of promoting hydration.
 For further information see: “Zoledronic
acid funded with Special Authority from September 1 2010”, BPJ 30 (Aug, 2010).
For further information see: “Zoledronic
acid funded with Special Authority from September 1 2010”, BPJ 30 (Aug, 2010).
Acknowledgement
Thank you to Kate Laidlow, Educator, Intravenous Nursing New Zealand for expert guidance in developing
this article.
References
- Caplan GA, Ward JA, Brennan NJ, et al. Hospital in the home: a randomised controlled trial. Med J Aust 1999;170:156-60.
- Corwin P, Toop L, G M, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home
compared with hospital. BMJ 2005;330:129-32.
- Aish H, Didsbury P, Cressey P, et al. Primary options for acute care: general practioners using their skills to manage “avoidable
admission” patients in the community NZ Med J 2003;116(1169):U326.
- Rathore MH. The unique issues of outpatient parenteral antimicrobial therapy in children and adolescents. Clin Infect
Dis 2010;51(Suppl 2):S209-15.
- Sheerin I, Allen G, Henare M, et al. Avoidable hospitalisations: potential for primary and public health initiatives
in Canterbury, New Zealand. NZ Med J 2006;119(1236):U2029.
- Ardagh M. How to achieve New Zealand's shorter stays in emergency deaprtments health target. NZ Med J 2010;123(1316):95-103.
- Howden BP, Grayson LM. Hospital-in-the-home treatment of infectious diseases. Med J Aust 2002;176:440-5.
- Stiver HG, Trosky SK, Cote DD, Oruck JL. Self-administration of intravenous antibiotics: an efficient, cost-effective
home care program. Can Med Assoc J 1982;127(3):207-11.
- Chambers S, Gallagher K, Metcalf S, Pithie A. Home intravenous antimicrobial service - twelve months expereince in
Christchurch. NZ Med J 2002;115:216-8.
- Barker G, Bryant L, Aish H. Primary options for acute care: cellulitis management in Counties Mankau DHB. NZ Faml
Phys 2006;33(1):39-45.
- Primary option for acute care. POAC information manual. Auckalnd, Counties Manukau and Waitemata. Available: www.primaryoptions.co.nz/page/information (Accessed
Jun, 2011). Auckland, 2011.
- Nottingham University Hospitals. Antibiotic guideline for the empirical treatment of sepsis. Available: www.nuh.nhs.uk/nch/antibiotics/,
2010.
- Primary Options for Acute Care. Guidelines for management of adult dehydration. Available on request from POAC. 2011
(Not yet released).
- Duggan C, Lasche J, McCarty M, et al. Oral rehydration solution for acute diarrhea prevents subsequent unscheduled
follow-up visits. Pediatrics 1999;104(e29).
- Royal College of Nursing. Standards for infusion therapy. Available: www.rcn.org.uk/__data/assets/pdf_file/0005/78593/002179.pdf (Accessed
Jun, 2011). The RCN IV therapy forum 2010.
- Centre for Disease Control and Prevention. 2011 Guidelines for the prevention of intravascular catheter-related infections.
Available at: www.cdc.gov/hicpac/BSI/BSI-guidelines-2011.html (Accessed
Aug, 2011) 2011.
- Medsafe. Zoledronic acid associated with adverse effects on renal function. Prescriber Update 2010;31(2):17.
- Medsafe. Alcasta: Zoledronic acid. Medicine Safety Datasheet. Available: www.medsafe.govt.nz/profs/datasheet/Zometaconcinf.pdf (Accessed
Jun, 2011). 2009.