Is your Practice measuring ankle-brachial pressure indices?
Peripheral artery disease is a significant risk factor for cardiovascular events and lower limb amputation. The prevalence
of peripheral artery disease is increased among older people, people who smoke and people who have diabetes. In New Zealand
there is limited epidemiological data on peripheral artery disease. However, it is likely that Māori and Pacific peoples
are more severely affected by peripheral artery disease compared with European New Zealanders, as they are known to have
significantly higher rates of cardiovascular disease in general.
The ankle-brachial pressure index (ABPI) is a non-invasive method for detecting or ruling-out the presence of peripheral
artery disease. ABPI is a calculation of the ratio of the patient’s systolic blood pressure at their ankle to the systolic
pressure in their arm. ABPI is generally between 1.0 – 1.4 in healthy people, i.e. the systolic pressure at the ankle
is greater than the systolic pressure at the arm. An abnormally low ABPI value (i.e. < 0.9) has a sensitivity of 79
– 95% and a specificity of approximately 95% for peripheral artery disease.1
Between one-third and one-half of patients with peripheral artery disease will have some evidence of coronary artery
or cerebrovascular disease.1 A meta-analysis of 16 studies involving over 48 000 patients without a history
of coronary artery disease, found that when ABPI indicated the presence of peripheral artery disease the risk of cardiovascular
mortality increased by over four times for males and approximately 3.5 times for females, compared with people with an
ABPI in the normal range.2
The majority of General Practitioners do not currently perform routine ABPI measurements – presumably because they do
not have access to the necessary equipment. When combined with a focused vascular examination, the ABPI is a useful tool
in primary care for stratifying a patient’s cardiovascular risk, and improving their management.
Ankle-brachial pressure index testing has multiple uses
A pedal pulse that is easily felt on examination effectively excludes peripheral artery disease. However, measuring
ABPI to detect peripheral artery disease is a more sensitive and replicable test compared to palpation of a pedal pulse,
especially in patients who are obese or who have significant oedema.1 Measurement of the ABPI can also provide
valuable clinical information without the need to refer the patient to a vascular laboratory.
ABPI is recommended for all patients who present with signs and symptoms suggestive of peripheral artery disease.
The physical examination of a patient with peripheral artery disease may reveal reduced or absent pedal pulses on palpation,
skin that is cool, shiny, hairless or thin, thickening of the nails, abnormal capillary refill time, pallor of distal
extremities on elevation, leg pain and tissue ulceration or necrosis.3 The classical initial symptom of peripheral artery
disease is intermittent claudication. This is a tight cramp-like pain in the muscles of the calf, thigh or buttock that
is reproduced with exercise and relieved within ten minutes of rest.3 However, only 10% of patients with peripheral
artery disease present with classical claudication and approximately 50% have atypical leg pain; the remainder of patients
are asymptomatic.3 Venous claudication, neurogenic claudication (spinal stenosis), popliteal artery entrapment,
Raynaud’s phenomenon and other vasospastic problems are differential diagnoses that may need to be considered in patients
with symptoms suggestive of claudication. Therefore ABPI testing is not only useful for detecting the presence of peripheral
artery disease, it is also helpful for ruling-out peripheral artery disease as a cause of symptoms in the lower limbs,
particularly in older patients.
ABPI provides an indication of disease severity and the urgency of referral. The presence of ischaemic
rest pain suggests increased severity of peripheral artery disease and an increased risk to the limb. Patients with ischaemic
rest pain often present with a burning pain in the arch or distal foot that occurs when their feet are elevated, e.g.
in bed, and resolves when they place their feet on the floor. An ABPI < 0.4 indicates the patient has critical limb
ischaemia.4 This is a potentially life-threatening condition characterised by severely reduced circulation,
ischaemic rest pain and tissue loss due to ulceration and/or gangrene.5 Due to severely impaired circulation,
5 – 10% of patients with peripheral artery disease will require surgical revascularisation to reduce the risk of amputation.5
ABPI is used to assess the safety of compression treatment when considering compression hosiery and
bandaging for patients with venous disease or ulceration. ABPI may still be performed as a confirmatory measure in patients
with a palpable pedal pulse, before applying compression hosiery or compression bandages, because of the risk of complications
developing in patients with undiagnosed peripheral artery disease.
ABPI is used to exclude peripheral artery disease in patients who are undergoing treatment that may
result in vascular complications, e.g. patients undergoing leg or foot surgery.5
Targeted use of ABPI in asymptomatic patients
There is currently insufficient evidence to recommend population screening for peripheral artery disease using ABPI.3 However,
international guidelines recommend that people who are at risk of developing artery disease (see below), be offered a
clinical assessment that includes an ABPI measurement.3, 5
Risk factors for peripheral artery disease include:3, 5
- Older age
- Smoking, past and present
- Diabetes
- Hyperlipidaemia
- Hypertension
- Reduced renal function (eGFR < 60 mL/min/1.73 m2 )
In particular, international guidelines recommend targeted testing for peripheral artery disease for the following groups:6
- All people aged between 50 and 69 years who smoke or have diabetes
- All people from age 70 years regardless of risk-factor status
- All people with a Framingham risk score > 10%
Current smokers are estimated to be almost four times as likely to develop peripheral artery disease as non-smokers.5 Over
half of all amputations due to peripheral artery disease are reported to occur in patients with diabetes.5
Performing ankle-brachial pressure index testing
The following equipment is recommended for measuring the ankle-brachial pressure index:8
- A hand-held portable Doppler device with a frequency of 8 – 10 MHz, although 5 MHz probes may be better for patients
with significant ankle oedema. Devices can be purchased for under $700* and training is generally provided
by the supplier. More expensive devices with LCD screen and printing options are also available.
- A sphygmomanometer
- Ultrasound transmission gel
NB: $700 is the approximate cost of the hand-held Doppler device.
A Doppler probe to attach to the device is also required which will cost an additional $700 approximately.
How to measure the ankle-brachial pressure index
For the purposes of excluding peripheral artery disease it is sufficient to perform only one ABPI measurement, i.e.
by dividing the systolic pressure detected at a single posterior tibial artery by the systolic brachial pressure of one
arm (see below). The diastolic pressure is not measured and is not required when measuring the ABPI.
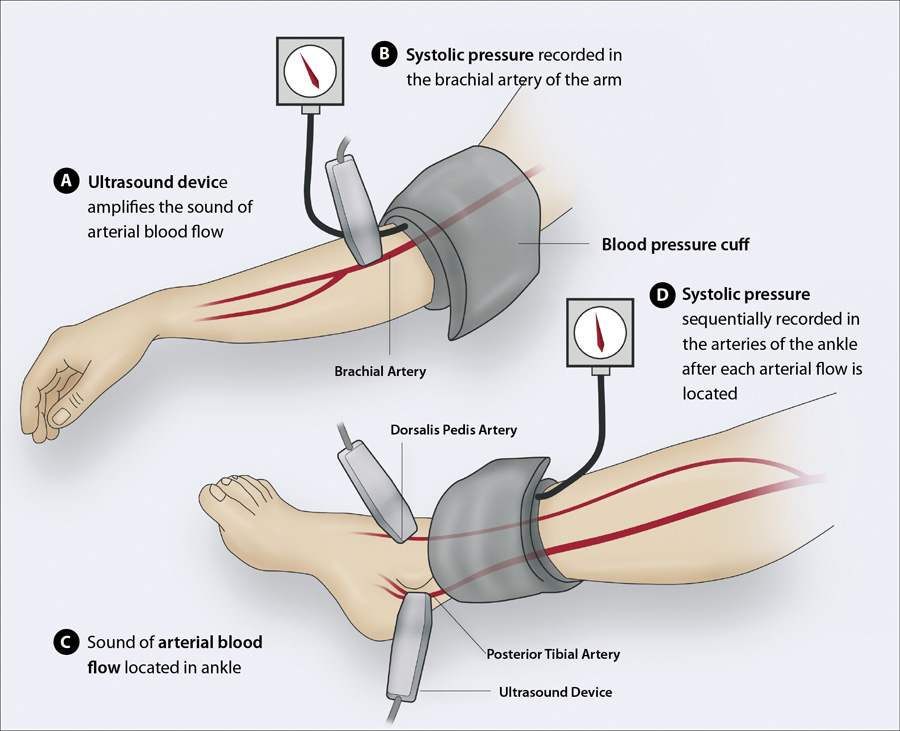
With the patient in a supine position (Figure 1):
- Place the blood pressure cuff approximately two to three centimetres above the antecubital fossa for the brachial
pressure and approximately five centimetres above the medial malleolus for the ankle pressure
- The Doppler probe should detect a clear arterial pulse before the cuff is inflated
- Inflate the cuff slowly until the systolic pressure is indicated by the disappearance of the Doppler sound. N.B.
This does not need to be highly precise as the ratio is calculated to a single decimal point.
- Divide the ankle systolic pressure detected at the posterior tibial artery by the brachial pressure
- If the patient’s ABPI is < 0.9 then this indicates they have peripheral artery disease and additional measurements
are recommended to increase the accuracy of the assessment of the disease severity:
- Divide the highest ankle systolic pressure in each of the posterior tibial and dorsalis pedis arteries* in both feet
by the highest brachial systolic pressure from each arm; the lowest resulting value is the patient’s overall ABPI.
* This measurement may not be possible in all patients as 12% of the general population has a congenital
absence of the dorsalis pedis pulse.8
The ABPI procedure may cause discomfort for patients with lower leg pain or cellulitis. If ulcers or wounds are present
on the ankle then a protective barrier, e.g. a plastic wrap, should be placed over the affected area before the cuff is
applied.8
Figure 1: Sequentially measuring the brachial systolic pressure and ankle systolic pressure
in the posterior tibial and dorsalis pedis arteries with a single hand-held Doppler ultrasound device
Interpreting the ankle-brachial index
An ABPI between 1.0 – 1.4 (Table 1) is sufficient to exclude peripheral artery disease in most
patients (see: “Limitations of ankle-brachial pressure index”). Referral to a vascular laboratory
should be considered for patients with an ABPI > 1.4, as this result is clinically inconclusive. In a patient with
a borderline APBI, i.e. 0.9, where there are additional reasons to suspect peripheral artery disease, e.g. symptoms and
risk factors, consider discussing the result with a vascular surgeon as further investigations, such as exercise testing,
may be recommended.
An ABPI of < 0.9 indicates significant occlusion in the arteries supplying the patient’s lower extremities and is
diagnostic for peripheral artery disease. The lower the patient’s ABPI, the more severe the disease, with an ABPI < 0.4
indicating critical limb ischaemia.4
In patients with an ABPI > 0.8 compression hosiery is considered safe.9 However, in patients with an
ABPI < 0.8, high compression hosiery (i.e. 30 – 40 mmHg at the ankle) is not recommended when treating lower limbs,
e.g. non-healing leg ulcers in patients with diabetes, due to the increased risk of skin necrosis.8 If ABPI is < 0.5,
compression hosiery should not be used.8
Table 1: Clinical interpretation of the ankle-brachial index (ABPI)1, 4, 5
| Ankle-brachial pressure index (ABPI) |
Clinical interpretation |
| > 1.4 |
Inconclusive due to non-compressible blood vessels |
| 1.0 – 1.4 |
Normal; peripheral artery disease can be excluded in most patients |
| 0.9 |
Borderline; discussion with a vascular surgeon may be appropriate depending on the patients symptoms and risk factors |
| < 0.9 |
Abnormal and diagnostic of peripheral artery disease |
| < 0.4 |
Critical limb ischaemia |

ABPI can be used as a marker of cardiovascular risk
A low ABPI, i.e. < 0.9, is an independent predictor of cardiovascular risk and measuring ABPI has been widely suggested
for the detection of subclinical disease in order to prevent cardiovascular mortality and stroke.1, 2, 7 For
some patients detection of a low ABPI may allow a more accurate estimation of cardiovascular risk than is provided solely
by traditional risk assessment tools, e.g. patients with no other history of cardiovascular disease.7 A meta-analysis
involving over 48 000 patients found that an ABPI ≤ 0.9 approximately doubled the risk of total mortality, cardiovascular
mortality and major coronary events across all Framingham risk categories assessed.2 For example, the overall
ten-year rate of cardiovascular mortality was 7.3% for males with an ABPI between 0.91 and 1.1, but 18.7% in males with
an ABPI ≤ 0.9.2
What to do when a patient is diagnosed with peripheral artery disease
After performing a vascular examination, criteria that would indicate an increased urgency of referral to a vascular
surgeon include:
- An ABPI < 0.5
- Known peripheral artery disease presenting with a new ulcer or area of necrotic tissue
- An ulcer that is not responding to treatment
- Intermittent claudication when walking for less than 200 m
- Young and otherwise healthy patients with claudication to rule-out rare causes, e.g. popliteal artery entrapment
Discussion with a vascular surgeon should also be considered when:
- There is doubt concerning the patient’s diagnosis
- There is uncertainty around the significance of an ABPI result
- There is doubt about the need for treatment or what treatment options are available

The limitations of ankle-brachial pressure index testing
- The Doppler device that is used in the measurement of ABPI indicates the velocity of blood flow and although this
is related to blood volume, it is not a measure of the amount of blood that peripheral tissues are receiving.
- The technique is unable to determine the exact location of a patient’s arterial stenosis or occlusion.
- ABPI can be falsely elevated in patients with calcification of the medial arteries, e.g. in some patients with diabetes,
renal dysfunction or rheumatoid arthritis.8
- Some patients with arterial stenosis may present with intermittent claudication and normal ankle pressures at rest.8 Referral
for vascular testing may be required for patients where there is reason to suspect the presence of peripheral artery
disease despite a normal or elevated ABPI being recorded.
Treatment of peripheral artery disease
The treatment of peripheral artery disease focuses on:
- Improving quality of life in symptomatic patients
- Reducing overall cardiovascular risk, which may have a small disease-modifying effect on peripheral artery disease
All patients with an ABPI < 0.9 have peripheral artery disease and are clinically assumed to have a 5-year cardiovascular
risk > 20%.10 Therefore the use of cardiovascular risk charts when performing routine cardiovascular risk
assessments in these patients is not necessary and management of cardiovascular risk factors should be intensive.10 The
modifiable and non-modifiable risk factors for peripheral artery disease are the same as those for other forms of cardiovascular
disease.5
Patients with peripheral artery disease will often have co-morbidities. An Australian study of patients in general practice
from 2008 – 2012 found that the prevalence of managed, i.e. known, co-morbidities in patients with peripheral artery disease
was: hypertension (10.7%), diabetes (8.0%), lipid disorders (3.9%) and ischaemic heart disease (3.7%).11
Lifestyle advice is the first-line treatment for peripheral artery disease
Some patients with peripheral artery disease may not associate their symptoms with their lifestyle, e.g. smoking or
a lack of exercise. Give patients lifestyle advice to address modifiable risk factors, which in turn is likely to improve
the symptoms of peripheral artery disease:5
- Smoking cessation
- Regular exercise
- Weight loss
- Eating a healthy and balanced diet
Smoking cessation advice and support should be given to all patients with peripheral artery disease who smoke.5 There
are relatively few robust studies investigating the direct benefits of smoking cessation on peripheral artery disease.
There is observational evidence suggesting that smoking cessation will improve mobility in patients with peripheral artery
disease.5 However, the strongest evidence for the benefits of smoking cessation in patients with peripheral
artery disease comes from cardiovascular outcomes. The excess cardiovascular risk of people who smoke is reported to be
halved within one year of cessation and be the same as non-smokers within five years.5 It can also be explained
to patients that continued smoking will decrease the effectiveness of other interventions such as exercise programmes
or surgery.
Patients are recommended to walk for twenty minutes per day and encouraged to exercise to the point
of maximal pain.5 Improvement, e.g. towards the goal of pain-free walking in patients with intermittent claudication,
should be assessed after three months and regularly thereafter.5 Patients with peripheral artery disease require
a structured programme of regular walking because people who participate in exercise programmes have been found to benefit
from improved limb function and general health. This is likely to be due to improved distal blood flow following the creation
of new collateral blood vessels stimulated by the production of growth factors, e.g. vascular endothelial growth factor,
and the release of vasodilating compounds, e.g. nitric oxide.12 Compliance with an exercise programme is likely
to be improved by supervision. Supervised exercise programmes involving walking three times a week on a treadmill have
been shown to provide greater benefit to patients with peripheral artery disease compared with unsupervised programmes.13 Where
supervised programmes are not accessible, suggesting that patients participate in group exercise programmes may improve
compliance and replicate the benefits of supervised exercise programmes.
Pharmacological treatment of peripheral artery disease itself is unproven
There is little evidence supporting the pharmacological treatment of peripheral artery disease itself. However, emerging
evidence suggests that angiotensin converting enzyme (ACE) inhibitors may improve walking ability in patients with intermittent
claudication. A meta-analysis of six studies comprising over 800 patients found that treatment with an ACE inhibitor improved
the maximum walking distance of patients with intermittent claudication by approximately 120 metres and improved pain-free
walking distance by approximately 75 metres.14 However, the ACE inhibitor with the greatest evidence of benefit
is ramipril, which is not currently available in New Zealand. It is unknown if the improvement in walking distance associated
with ramipril is due to a class effect of ACE inhibitors or whether it is specific to this medicine. The use of ACE inhibitors
has not been shown to have a significant effect on ABPI, although this may be due to the limitations of ABPI testing.14 Additional
guidance on this issue will be published when more evidence is available.
Pentoxifylline (oxypentifylline) is a vasoactive medicine that has been used to improve blood flow in patients with
peripheral artery disease by decreasing blood viscosity. It is partially subsidised in New Zealand, but is rarely used.
In the United Kingdom the use of pentoxifylline is not recommended for the treatment of intermittent claudication in patients
with peripheral artery disease, on the basis of lack of evidence of clinical and cost-effectiveness.15
Pharmacological reduction of cardiovascular risk is recommended for all patients
Patients with peripheral artery disease require pharmacological treatments to reduce their cardiovascular risk:
- Antiplatelet treatment with either aspirin or clopidogrel (depending on the patient’s cardiovascular
history and presence of co-morbidities) is recommended for prevention of vascular ischaemic events. Antiplatelet treatment
reduces the risk of serious vascular events by approximately one-quarter in patients with peripheral artery disease.16
- Statins are recommended for all patients with peripheral artery disease, unless contraindicated.
NICE guidelines report a 17.6% reduction in cardiovascular events for patients with peripheral artery disease taking
simvastatin with a total cholesterol > 3.5 mmol/L.5 Statin use may also result in atherosclerotic plaque stabilisation
and even plaque regression independently of their lipid-lowering ability.
- Hypertension should be treated to a target of 130/80 mmHg.10 Dietary salt intake should
be restricted.
- HbA1c target for patients with diabetes and peripheral artery disease should be ≤ 50 –
55 mmol/L (or as individually agreed, depending on other clinical factors).10
- Renal function should be monitored regularly, e.g. annually, in patients with peripheral artery disease.
Microalbuminuria is the earliest sign of diabetic kidney disease.10
Beta-blockers may be cautiously continued in patients with peripheral artery disease where they are
clinically indicated. Contrary to historical concern, a Cochrane review of six studies with a small sample of 119 patients
found no evidence that the use of beta-blockers adversely affected walking distance, calf blood flow or vascular resistance
in patients with peripheral artery disease.17
 For further information on pharmacological treatment recommendations for hypertension
and diabetes, see: “Hypertension in adults: the silent killer”, BPJ 54
(Aug, 2013) and “Improving glycaemic control in people with type 2 diabetes”,
BPJ 53 (Jun, 2013).
For further information on pharmacological treatment recommendations for hypertension
and diabetes, see: “Hypertension in adults: the silent killer”, BPJ 54
(Aug, 2013) and “Improving glycaemic control in people with type 2 diabetes”,
BPJ 53 (Jun, 2013).
Referral may be required if interventions are unsuccessful
Vascular surgeons can provide advice and suggestions of additional treatment options at any stage during the patient’s
management. If smoking cessation, exercise, weight loss and pharmacological reduction of CVD risk have not been effective
in improving the patient’s symptoms within six months, then patients with peripheral artery disease should be referred
to a vascular surgeon to discuss ongoing management of their condition, including a tailored exercise programme. Surgical
procedures are performed on relatively few patients compared to the number of people diagnosed with peripheral artery
disease. Vascular imaging, functional testing and surveillance programmes may be considered before more invasive procedures
such as angioplasty, stent placement or revascularisation are considered.
Acknowledgement
Thank you to Professor Andre van Rij, Vascular Surgeon, Ralph Barnett Professor of Surgery, Department
of Surgical Sciences, Dunedin School of Medicine, University of Otago for expert review of this article.