Losing touch: diabetic peripheral neuropathy
Diabetes is one of the most common causes of neuropathy, with up to 50% of people with type 2 diabetes eventually developing
some degree of peripheral neuropathy.1 Diabetes can affect many different elements of the peripheral nervous
system and result in neuropathies of several types, characterised by a variety of symptoms, including sensory disturbance,
autonomic dysfunction and weakness (see “Classification of neuropathies in people with diabetes”).
It is estimated that 90% of people with diabetic peripheral neuropathy have symmetric distal polyneuropathy, where multiple
nerve groups are affected.1 This often occurs in combination with autonomic neuropathy.1, 2 Focal
and multifocal neuropathies, affecting one nerve or nerve group (mononeuropathies), e.g. cranial nerve palsies or radiculoneuropathies,
occur less often. It is important to note that a person with diabetes may have more than one form of neuropathy, e.g.
both symmetric distal polyneuropathy and carpal tunnel syndrome (which occurs in up to one-third of people with diabetes).2,
3, 4
Many mechanisms are thought to be involved in producing the damage to nerves in people with diabetes, and this is an
ongoing area of research.5 In diabetic neuropathy, a number of metabolic and vascular changes interconnect
to cause damage to nerve cells in a similar way to that seen in diabetic retinopathy and nephropathy, with the primary
underlying factor being hyperglycaemia.5, 6 Changes include increased oxidative stress, a build-up of glycation
end-products, increased activity of the polyol pathway, activation of pro-inflammatory mechanisms and ischaemia.5,
6 These processes have direct and indirect adverse effects, not only on neurons and Schwann cells, but also on
the vascular tissue of the blood vessels that supply the nerves.5 All types of nerve fibres, e.g. sensory,
autonomic, motor, both myelinated and unmyelinated, are adversely affected in people with diabetes.5
As with the other microvascular complications associated with diabetes, the risk of developing neuropathy is proportional
to both the magnitude and duration of hyperglycaemia.7 The development of diabetic neuropathy is therefore
less likely in people with optimal long-term control of HbA1c levels (< 55 mmol/mol).
Additional modifiable risk factors for the development of diabetic peripheral neuropathy include smoking, hypertension,
obesity and dyslipidaemia.7 Increasing age, a family history of neuropathy and the duration of diabetes are
non-modifiable risk factors.7
Although in a person with diabetes it is most likely that this condition will be responsible for the neuropathy, other
diagnostic possibilities should be considered, including medicines, systemic conditions, infections, autoimmune disorders,
toxins, trauma and inherited conditions.8 Neuropathy due to vitamin B12 deficiency, uraemia or hypothyroidism
are known to occur more often in people with diabetes.9 Chronic inflammatory demyelinating polyneuropathy
(CIDP) may also be more common in people with diabetes. Diabetic peripheral neuropathy, therefore, is often regarded
as a diagnosis of exclusion.9
 See: “Alternative causes for peripheral neuropathy in a person with
diabetes”.
See: “Alternative causes for peripheral neuropathy in a person with
diabetes”.
For most people with diabetic peripheral neuropathy, the outcome that is most feared is “diabetic foot”, where the
loss of protective sensation, often accompanied by reduced perfusion from arterial disease, increases the risk of ulceration,
infection and, ultimately amputation. In addition, diabetic peripheral neuropathy can have a significant impact on a
patient’s quality of life due to its negative impact on sleep, daily activities, independence and mood, and also due
to an increased risk of falls and fractures.4,10
The risk of amputation in a patient with neuropathy increases 1.7-fold, further increasing to 12-fold if there is also
deformity of the foot (which may be a consequence of the neuropathy) and up to 36-fold if the patient has a previous
history of ulceration.4 It is estimated that at least half the foot ulcers that occur in people with diabetic
neuropathy could be prevented by appropriate management and increasing the patient’s understanding of their condition.3 This
involves considering the principles of cultural competency, with health literacy being an important component of this.
“I never noticed that, Doc!” – Recognising and diagnosing diabetic neuropathy in a primary care setting
Of the estimated 50% of people with diabetes who develop peripheral neuropathy, up to half will be asymptomatic or
have numbness as their only symptom;3 they literally cannot feel it coming. Diabetic peripheral neuropathy
is usually insidious in onset and therefore assessment for neuropathy must be an active part of the routine follow-up
of all people with diabetes. Patients need to be asked about the presence of symptoms, such as numbness, tingling or
pain, and examined for signs of neuropathy, including specific sensory testing (e.g. monofilament testing, tuning fork
tests), which may detect patients with diabetic peripheral neuropathy who are asymptomatic. An absence of symptoms, however,
does not mean an absence of neuropathy.9
There is no readily-available clinical gold-standard test for diagnosing peripheral neuropathy. The diagnosis is based
on clinical suspicion, generated by a combination of findings from the history and examination, followed by the exclusion
of other potential causes.4 If a patient with diabetes has retinopathy or nephropathy it is likely that they
will also have neuropathy.2
What are the symptoms of peripheral neuropathy?
Symmetric distal polyneuropathy, the most common form of diabetic peripheral neuropathy, usually has
a mild, insidious onset, with a predominance of sensory symptoms over motor symptoms.4 It is present at the
time of diagnosis of diabetes in up to 10% of patients with type 2 diabetes.3
The symptoms vary widely, depending on the specific pattern of damage to nerve fibres of different size and function.
A loss of pain sensation and the ability to perceive changes in temperature tend to be the result of damage to small
sensory fibres (Type-C). The loss of sensation to touch, vibration, proprioception and motor innervation of the intrinsic
muscles of the foot from damage to large fibres (Type-A).2, 4, 11 Neuropathic symptoms are defined as positive
(“painful”) or negative (“non-painful”).3, 12
Positive symptoms include a sensation of burning or knife-like pain, electrical sensations, squeezing,
constricting, freezing or throbbing and allodynia – pain provoked by a stimulus that is not normally painful, e.g. stroking
the skin.3, 12 Pain may be highly variable in presentation, but patients are usually more prone to nocturnal
exacerbations.3 Positive symptoms appear to stem from increased uninhibited sensory firing from the damaged
nerve fibres.
Negative symptoms include sensations of tingling, swelling, prickling, numbness, a feeling of “walking
on cotton wool” or that the limb is “asleep” or “dead”.3, 12 Negative symptoms are thought to be generally
due to reduced signalling from damaged nerves. Patients with negative symptoms are at higher risk of foot ulceration
due to the lack of protective sensation.
The sensory symptoms usually first appear in the toes and gradually progress proximally in a “stocking distribution”
to involve the feet and legs.6 This is because the sensory nerves with the longest axons are affected first
and the neuropathy is often termed “length-dependent”.6, 7 Patients may also develop symptoms in the fingers
which gradually involve the hand, however, this is uncommon unless the symptoms in the legs have progressed to mid-thigh
level, typically in people with later-stage diabetic neuropathy.2 Generally, the symptoms have a symmetrical
distribution and typically there are nocturnal exacerbations of painful sensory symptoms.6
Motor symptoms such as atrophy, weakness and unsteadiness are also potential manifestations of diabetic peripheral
neuropathy, although traditionally these were not considered symptoms of the condition and they are more common later
in the disease course.3, 13 The development of unsteadiness and ataxia generally occur due to abnormalities
of proprioception and muscle sensory function.4 Severe sensory ataxia is not a feature of diabetic peripheral
neuropathy and if present should prompt consideration of alternative causes.
 Best Practice Tip: If a patient with diabetes has peripheral
symptoms that are more prominent in the upper limbs than the lower limbs then an alternative explanation for the sensory
changes in the upper limb should be considered.2
Best Practice Tip: If a patient with diabetes has peripheral
symptoms that are more prominent in the upper limbs than the lower limbs then an alternative explanation for the sensory
changes in the upper limb should be considered.2
Autonomic neuropathic dysfunction affecting both sympathetic and parasympathetic functions can also
occur in people with diabetes, with or without sensorimotor neuropathy. Typically dysfunction can involve the cardiovascular,
gastrointestinal, genitourinary, sudomotor (control of the sweat glands) and ocular systems.14 Some problems,
e.g. erectile dysfunction, are often not reported, so should be enquired about at least once per year.15
Autonomic symptoms therefore vary widely but may include:14
- Cardiovascular – resting tachycardia, orthostatic hypotension, exercise intolerance, silent myocardial ischaemia
- Gastrointestinal – symptoms of gastroparesis (early satiety, bloating, vomiting, digestive problems, erratic glucose
control following meals),15 diarrhoea, constipation, faecal incontinence
- Genitourinary – bladder-voiding problems (e.g. neurogenic bladder), erectile dysfunction, retrograde ejaculation,
female sexual dysfunction (e.g. loss of vaginal lubrication)
- Metabolic – hypoglycaemia unawareness, hypoglycaemic-associated autonomic failure
- Sudomotor – excessive sweating in the upper body and reduced sweating in the legs and feet, heat intolerance, localised
sweating over the face and neck after meals (gustatory sweating), dry, flaky, cracked skin on the feet and increased
formation of callus (caused by reduced sweating in the feet)7, 16
- Ocular – pupillomotor function impairment (e.g. decreased diameter of dark-adapted pupil), Argyll-Robertson pupil
(small pupil that constricts poorly to light, but rapidly to a close object)
 Best Practice Tip: If a patient with diabetes has a peripheral
neuropathy that is mild, but prominent or severe autonomic symptoms, other causes for the autonomic neuropathy should
be considered, e.g. amyloid neuropathy.2
Best Practice Tip: If a patient with diabetes has a peripheral
neuropathy that is mild, but prominent or severe autonomic symptoms, other causes for the autonomic neuropathy should
be considered, e.g. amyloid neuropathy.2
Hyperglycaemic neuropathy (acute sensory neuropathy); in contrast to many patients with symmetric
distal neuropathy, patients with hyperglycaemic neuropathy will typically have a relatively normal physical examination.3 There
may be loss of light touch sensation, allodynia may be present on sensory testing and, occasionally, ankle reflexes will
be reduced. Motor function will usually be normal.3
”The sole issue”: examining for diabetic peripheral neuropathy
Many patients with diabetes have asymptomatic peripheral neuropathy and those that are symptomatic tend to have variable
symptoms which are reported to have a relatively poor diagnostic accuracy. Therefore, a diagnosis may rely heavily on
the clinical signs detected on examination.13 The most common causes of foot ulceration in a patient with
diabetes are peripheral neuropathy, deformity of the foot and external trauma, with peripheral arterial disease and peripheral
oedema also having a significant contribution.17
Examination of a patient with suspected diabetic neuropathy should include:4, 18
- A general inspection of the feet and the patient’s footwear
- Musculoskeletal assessment for deformity (including Charcot arthropathy)
- Neurological assessment
- Vascular assessment of the feet, and assessment of the heart rate and blood pressure (lying/sitting and standing)
General inspection of the feet
Examine both feet and check the condition of the skin, particularly looking for erythematous areas, dryness, flakiness,
thickness, cracking, callus formation, infection and ulceration.18 Dermatological changes, such as dry or
scaly skin, may be secondary to a degree of autonomic dysfunction which can begin distally. There may also be abnormalities
of sweating or circulatory instability in the feet, e.g. a hot or cold foot.13 Heavy callus formation over
the pressure points of the foot and signs of localised rubbing or friction, blisters or erythema can also be an indication
of inappropriate footwear.18 Foot ulcers are not caused by neuropathy alone but can occur without injury
once hard callus is present over pressure points. If a patient has a loss of sensation in the foot there will be prolonged
and increased forces on the callused areas which then increases the risk of tissue breakdown and ulceration.11
Musculoskeletal assessment
Foot deformity has a significant role in the development of pressure points in the foot which predispose it to ulceration.
There may be prominence of the metatarsal heads and other bony prominences that increase the risk of skin breakdown.
Callus formation frequently results in a deformity sufficient to lead to ulceration. Callus is most commonly formed on
the plantar surface beneath the first metatarsal head due to focal pressure during walking.11 Hyperextension
of the metatarsal phalangeal joints with flexion of the interphalangeal joints can result in claw toes, while extension
at the distal phalangeal joints causes hammer toes.18 Extreme deformity can develop very acutely in a neuropathic
foot, usually in the midfoot, and cause a “Charcot foot” (see: “Charcot arthropathy”).11 Signs
of motor involvement may include muscle atrophy, particularly “guttering” between the metatarsals, and muscle weakness
beginning with weakness of toe dorsiflexion followed by weakness of foot dorsiflexion.13, 18
Neurological assessment
The classic pattern of sensory loss in a patient with symmetric distal polyneuropathy is a length dependent, non-dermatomal
distal loss affecting all modalities, e.g. light touch, pin prick and temperature.2, 9 This is referred to
as a “sock or stocking” distribution that may extend to the mid-calf.2 In severe cases it may extend further
up the leg and even rarely on to the trunk, or involve the upper limbs, beginning in the fingers (a “glove” distribution).1,
2
In a patient with diabetes, sensory loss is most often determined with the use of monofilament testing. An ability
to detect pain and light touch can be assessed with the use of a sharp examination pin or sterile needle and a wisp of
cotton wool. An impairment of the perception of vibration, assessed with a 128 Hz tuning fork, is often regarded as the
first objective evidence of symmetric distal polyneuropathy.4
The deep tendon reflexes may be reduced or absent, particularly those at the ankle. Some experts regard the loss of
ankle reflexes as a cardinal sign of symmetric distal polyneuropathy, however, other possible causes such as an S1 radiculopathy,
other focal neuropathies and a tendency for an age-related decrease in the reflexes must also be considered.2, 13
 Best Practice Tip: The presence of asymmetrical neurological
symptoms or findings on examination (e.g. loss of the ankle jerk in one leg only) is likely to suggest an alternative
cause for the symptoms.1 More marked symmetrical proximal weakness can suggest an alternative type of neuropathy,
e.g. CIDP.4
Best Practice Tip: The presence of asymmetrical neurological
symptoms or findings on examination (e.g. loss of the ankle jerk in one leg only) is likely to suggest an alternative
cause for the symptoms.1 More marked symmetrical proximal weakness can suggest an alternative type of neuropathy,
e.g. CIDP.4
Vascular assessment
Peripheral arterial disease is an important risk factor for the development of ulceration in the lower limbs. It is
estimated to be a significant underlying cause in approximately one-third of patients with foot ulcers.18 The
patient’s foot should be palpated to determine the presence and character of the posterior tibial and dorsalis pedis
pulses. Further investigation using the ankle-brachial pressure index (ABPI) can provide additional information and help
determine the patient’s risk of ulceration and the need for referral.18 N.B. ABPI can be falsely elevated
in some patients with diabetes, due to medial artery calcification.21
On examination, patients who have autonomic neuropathy affecting the cardiovascular system may be found to have a resting
tachycardia and orthostatic hypotension, and may report reduced exercise tolerance. Orthostatic hypotension in particular
can increase the risk of falls and cardiovascular autonomic neuropathy is associated with an increased risk of cardiovascular
morbidity and mortality.14
 For further information, see: “The
ankle-brachial pressure index”, BPJ 60 (Apr, 2014).
For further information, see: “The
ankle-brachial pressure index”, BPJ 60 (Apr, 2014).
Laboratory investigations may help confirm the underlying cause
Laboratory investigations are not generally required for the diagnosis of diabetic peripheral neuropathy, however,
they are usually requested to help exclude other causes of neuropathy. Initial investigations would usually include a
full blood count, CRP, HbA1c, liver function tests, creatinine clearance, vitamin B12, folate and thyroid-stimulating
hormone tests.8 Additional tests, may be considered if there is clinical suspicion of a specific potential
cause. Generally these tests would only be requested in consultation with a relevant specialist, such as an Endocrinologist
or Neurologist, e.g. cerebrospinal fluid (CSF) analysis to evaluate for CIDP or genetic testing if hereditary peripheral
neuropathy is suspected.8 Investigation for a paraprotein may also be recommended.
Referral for electrodiagnostic testing is rarely required, but may be considered for patients with
atypical features of neuropathy, e.g. onset of symptoms in the hands, proximal rather than distal weakness or marked
sensory ataxia.
Charcot arthropathy
Charot arthropathy is a neuropathic arthropathy resulting in degeneration of the stress bearing part of a joint, usually
affecting the foot and ankle. The pattern of bone destruction was first described in 1868 by Jean-Marie Charcot, although
it was not until 1936 that this neuroarthopathy was associated with diabetes.7 It is more characteristically
found in people with long-standing, often poorly controlled diabetes and is estimated to affect up to 10% of people
with neuropathy.7
There are two theories proposed for the development of Charcot arthropathy: neurotraumatic involving impaired proprioception,
with overuse injuries of insensate joints; and neurovascular, focusing on autonomic dysfunction, with increased blood
flow (through arterial-venous shunting) and an imbalance of bone destruction and synthesis.7
The classic deformity (“Charcot foot”) in a patient with neuropathy is collapse of the midfoot (the tarsometatarsal
joint), giving a flat appearance termed a “rocker bottom” foot.19 The loss of normal architecture in the
foot (loss of the medial arch, abnormal foot abduction) results in deformities that cause new pressure points which
may lead to ulceration, infections and amputation.7, 19 This, however, represents a late stage of the condition
and patients may present acutely much earlier before any deformity develops, with a hot, swollen, red foot with little
or no pain.7, 19 The foot pulses are usually easily palpable (often bounding), the foot veins may be distended
and swelling may extend up to the calf. The differential diagnosis at this stage includes infection (e.g. cellulitis
or osteomyelitis), deep venous thrombosis and acute gout.7, 19 Suspected acute presentation of Charcot arthropathy
is considered an emergency, and patients should be promptly referred to a specialist service.
Plain x-rays may be normal in the early stages of the condition, but with time may resemble “osteoarthritis with a
vengeance”. MRI can be helpful when plain x-rays are normal and can also assist with differentiating between a Charcot
joint and osteomyelitis, although making this distinction can be challenging.19
Management of a patient with Charcot arthropathy will depend on the stage and severity of the condition and if ulceration
or infection is present. Prevention is the optimal treatment, so early identification of patients who may be at risk
(e.g. older, diabetes for > 10 years, loss of protective sensation in the foot) is important.7 Treatment
initially includes rest and restrictions on weight-bearing, with immobilisation required in a total contact cast or
“moon boot” for some patients. The foot may need to be kept non-weightbearing for up to six months to minimise the development
of deformity. Surgery may be required to stabilise the foot, once the acute phase of the arthropathy has settled, with
the aim of reducing the prominence of pressure points and to allow ulceration, if present, to heal.7, 19 Arthrodesis
of the affected joints or amputation can be required in patients with severe deformity. Once the acute symptoms have
been stabilised, ongoing protective footwear will be required, often for life, which can range from custom inserts in
the shoes to various types of braces or walking boots.19
Sensory testing in primary care
There are a range of defined clinical tests that are used to assess sensory loss in a patient with suspected peripheral
neuropathy – their degrees of sensitivity, specificity, complexity and practicality vary. The most practical test used
in a primary care setting is monofilament testing, often with the addition of an assessment for the presence of vibration
sensation.2 It is widely reported that the loss of sensation, tested with a 10 g monofilament, is strongly
associated with the subsequent development of ulceration.18
Monofilament sensory testing uses a 10 g monofilament to assess a patient’s ability to feel light pressure
at a number of separate sites on the foot. The New Zealand Society for the Study of Diabetes guidelines suggest the examination
of 12 sites in total – six on each foot (Figure 1), although some clinicians believe that fewer sites are required, e.g.
four sites on each foot.18, 20 If the patient cannot detect the light pressure at more than one of the designated
testing sites, then loss of protective sensation is deemed to be present.20
To perform the test the patient is placed supine with bare feet (or their feet raised on a stool in front of the clinician).
The use of the filament should be demonstrated to the patient on their upper arm. Ask them to close their eyes and say
“yes” when they can feel the filament. The filament should then be placed against the foot, avoiding areas of callus if
possible, and pressed until the patient indicates they can feel it, or until the filament bows (Figure 1). The filament
should be pressed against the foot slowly over three seconds, not tapped. Site selection should be random and not predictable
by the patient.
N.B. It is recommended that a monofilament is not used on more than ten patients in 24 hours, as they may buckle. The
monofilament should also be replaced on a regular basis to ensure it still has a 10 g pressure. In addition, the monofilament
should be cleaned with alcohol after each use.
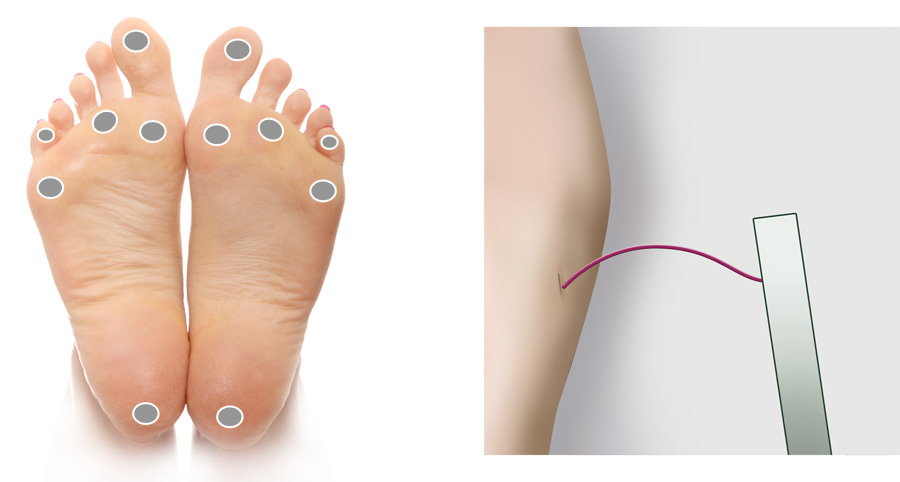
Figure 1: Recommended sites for cutaneous sensory pressure perception testing using a
monofilament. Monofilament bent to form a C shape.
Managing diabetic neuropathy in a primary care setting
The primary goal of treatment of diabetic neuropathy is reduction of the patient’s symptoms to a tolerable level and
prevention of further nerve damage. There is no specific treatment that can reverse nervous system damage in people with
diabetic peripheral neuropathy, but good glycaemic control may stabilise or even improve peripheral neuropathy over the
long-term.6 This reinforces the importance of ensuring people with diabetes have been provided with the tools
to understand their condition and their ability to self-manage. Management beyond glycaemic control is aimed at controlling
symptoms, particularly pain, and improving the patient’s quality of life. Protecting insensate feet from trauma is also
an important part of the management to avoid the development of ulcers.
Managing glycaemic levels can prevent further damage and control pain
Research has indicated that neuropathic pain in people with both sensory and sensorimotor diabetic neuropathy is associated
with periods of erratic glycaemic control.3 Stabilising and reducing glycaemic levels will benefit the majority
of patients, and will help to prevent further nerve damage.6 There is some evidence that optimal control of
glycaemic levels may improve symptoms over time,6 but this must be weighed against the increased risk from
hypoglycaemia and other serious adverse effects.
In people with acute sensory neuropathy, stabilising glycaemic levels is the primary goal of treatment.
Once stable glycaemia is achieved, severe symptoms will typically resolve in less than 12 months.3 Reducing
the overall glycaemic level is also important to prevent the development of chronic forms of neuropathy and other sequelae
associated with hyperglycaemia.

Neuropathy Disability Score
A modified form of the Neuropathy Disability Score (NDS) is a relatively simple, quick clinical assessment tool that
aims to combine a number of clinical tests to provide an assessment of the risk of neuropathic ulceration.3, 17
The clinical tests used in the modified NDS are:3
- Vibration perception threshold – Using a 128-Hz tuning fork, can the patient distinguish between
vibration/no vibration when the tuning fork is applied to the apex of the big toe? (score 0 if normal, 1 if abnormal)
- Temperature perception – Using the tuning fork and a beaker of ice or warm water, can the patient
distinguish temperature on the dorsum of the foot? (score 0 if normal, 1 if abnormal)
- Pin prick testing – Using a sharp single use neurological examination pin applied proximally to
the big toe nail, with just enough pressure to deform the skin, can the patient distinguish between sharp and not sharp?
(score 0 if normal, 1 if abnormal)
- Achilles tendon reflex – Is the reflex present (score =0), present with reinforcement (score = 1)
or absent (score = 2)?
Both feet should be tested and scored independently, and the results added together. The maximum score for the modified
NDS is 10, indicating a complete loss of all sensory modalities and absent reflexes. A score of six or more has been
found to indicate an increased risk of foot ulceration.3
Managing neuropathic pain
The pharmacological management of pain secondary to diabetic neuropathy can be challenging due to the multiple potential
underlying causes of the pain, and the range and severity of symptoms.23 Non-pharmacological methods, e.g.
exercise, should be trialled alongside medicines for the treatment of neuropathic pain.
Mild neuropathic pain may respond to paracetamol or NSAIDs
Paracetamol or a non-steroidal anti-inflammatory (NSAID) can be considered for patients with mild neuropathic pain.24 NSAIDs
should be used with caution in people with renal impairment, particularly if they are taking an angiotensin converting
enzyme inhibitor (ACEI) or angiotension receptor blocker (ARB).
 For further information, see: “NSAIDs:
making safer treatment choices”, BPJ 55 (Oct, 2014).
For further information, see: “NSAIDs:
making safer treatment choices”, BPJ 55 (Oct, 2014).
Consider the addition of a tricyclic antidepressant or an anticonvulsant
Amitriptyline, nortriptyline, gabapentin, pregabalin or duloxetine are all used in the management of moderate to severe
neuropathic pain.6, 23, 24 However, the treatment of neuropathic pain remains an unapproved indication for
tricyclic antidepressants (TCA). Gabapentin requires Special Authority approval for subsidy, following a trial of treatment
with a TCA, which has failed due to lack of efficacy or the patient is unable to tolerate the adverse effects.24 Duloxetine
or pregabalin are not currently subsidised in New Zealand.
The choice of treatment should be made after the usual consideration of contraindications, potential adverse effects,
interactions with other medicines, co-morbidities, potential benefits for co-morbid conditions (e.g. using an antidepressant
as a first choice in a person with diabetes and depression) as well as the patient’s preference.23
Topical treatment with capsaicin cream, 0.075%, can be considered for people with relatively localised neuropathic pain
who do not wish to take, or cannot tolerate, oral treatments.23 A Cochrane review of capsaicin cream (0.075%),
however, suggested that it had little meaningful effect in people with neuropathic pain.25
Consider adding an opioid if pain is not controlled
If the pain has not been controlled with a combination of paracetamol, NSAID, a TCA or an anticonvulsant such as gabapentin,
consider the addition of a weak opioid, e.g. codeine.24 Short-term use of tramadol can also be effective for
neuropathic pain, as an alternative to codeine.26 Tramadol can be considered for acute breakthrough pain if
required, but should not be used long-term without specialist consultation, or as a first-line monotherapy.23 Similar
restrictions apply to the use of strong opioids, such as morphine.24
There is insufficient data to recommend one opioid over another, so the choice should be made based on potency, adverse
effects, likelihood of misuse and/or in consultation with a relevant specialist.
 For dosing information for medicines used in neuropathic pain, refer to the New
Zealand Formulary: www.nzf.org
For dosing information for medicines used in neuropathic pain, refer to the New
Zealand Formulary: www.nzf.org
Exercise may be beneficial for neuropathic pain and peripheral neuropathy in general
There is evidence that exercise combining strength and aerobic activities is beneficial in reducing neuropathic pain,
as well as improving function in patients experiencing numbness, weakness and poor balance as a result of diabetic peripheral
neuropathy.27 In addition, exercise is a beneficial lifestyle intervention for patients with diabetes in general
and can help to prevent, or delay, diabetic peripheral neuropathy.27
It is thought that the most beneficial types of exercise for patients with peripheral neuropathy include strength-stability
(e.g. Tai Chi) and aerobic (e.g. walking) activities. Routine exercise has been shown to alleviate neuropathic pain, increase
plantar sensation, increase the ability to detect vibrations and improve trunk and ankle proprioception. The exact mechanism
by which exercise reduces neuropathic pain requires further investigation, but is thought to involve glial cell activation
and the release of noradrenaline and cytokines.27 Other benefits of exercise include enhanced macro- and micro-vascular
health (e.g. improved endothelial function and blood flow, reduced vasoconstriction), reduced risk of hypertension, atherosclerosis
and other cardiovascular conditions, increased muscle strength and reduced glycaemic levels.27
Managing autonomic neuropathic symptoms
Autonomic neuropathic symptoms are likely to be present in many patients with diabetic peripheral neuropathy. Features
of diabetic autonomic neuropathy can relate to one or more organ systems, e.g. cardiovascular, gastrointestinal, genitourinary,
sudomotor or ocular.14 Therefore management is individual, depending on which symptoms are present, but always
involves maintaining optimal control of diabetes.
When to refer patients with neuropathy
Patients with atypical features or who fail to respond to management strategies should be referred to a Neurologist
for further investigation. This includes patients with:4, 23
- Pronounced asymmetry of the neurologic deficits
- Predominant motor deficits, mononeuropathy or cranial nerve involvement
- Rapid development or progression of neuropathic impairment
- Progression of the neuropathy despite optimal glycaemic control
- Symptoms arising in the upper limbs
- Proximal weakness
- Significant sensory ataxia
- Family history of non-diabetic neuropathy
- Pain that is difficult to manage, limiting the patient’s lifestyle and daily activities or if their underlying health
has deteriorated as a result
Alternative causes of peripheral neuropathy in a person with diabetes

Diabetes is the primary cause of peripheral neuropathy in more than 90% of people with diabetes who develop peripheral
neuropathy.3 However, it is important in any patient with suspected diabetic neuropathy to ensure that diabetes,
and not an alternative condition, is causing the neuropathy.
There are a wide range of conditions, genetic abnormalities and environmental factors that can cause damage to the peripheral
nervous system. Peripheral neuropathy can be broadly classified into two groups: acquired or inherited. Acquired neuropathies
are more common and more likely to be encountered in primary care. They generally arise from three sources; physical trauma,
systemic disease or infections and autoimmune conditions.
Clinically there are few differentiating symptoms between the various causes of neuropathy. In some situations, there
will be a clear cause apparent in the patient’s history, e.g. a history of alcohol misuse or recent trauma. Patients who
have any mononeuropathy, evidence of a neuropathy with an asymmetrical distribution or an acute onset of symptoms are
more likely to have a cause other than diabetes for their neuropathy – although diabetes can cause isolated mononeuropathies,
e.g. a third cranial nerve palsy or lumbosacral radiculoplexus neuropathy (diabetic amyotrophy).
Acquired neuropathy
Traumatic neuropathy
Physical injury is a common cause of peripheral nerve damage and, if present in the history, is the most likely cause
of a neuropathy. Traumatic neuropathy occurs when the nerve is partially or completely severed, crushed, compressed or
stretched during an injury.
Referral will often be required for surgical management or rehabilitation, and symptomatic treatment of pain may also
be required.
Autoimmune and infectious neuropathy
Neuropathy can develop as a result of the inflammatory response of the body to many immune triggers, including infection.
A classic example of this in an acute illness is Guillain-Barre syndrome.28 CIDP may be considered a more
chronic version of a similar disease process which, although relatively rare, may be over represented in people with diabetes.9 CIDP
should be suspected if a patient with diabetic neuropathy has proximal or both proximal and distal weakness, early or
marked upper limb involvement, severe sensory ataxia or continued rapid progression despite reasonable glycaemic control.
The patient should be referred to secondary care and further testing with electrodiagnosis and CSF protein levels undertaken.
In some cases of CIDP, immunomodulatory therapy can produce a rapid and substantial improvement so making an accurate
and early diagnosis is important.9
Viruses and bacteria can damage nerve tissue, usually sensory fibres, leading to a painful neuropathy. The most common
example of this is herpes varicella-zoster. Less commonly, Epstein-Barr virus, cytomegalovirus and herpes simplex virus
can also cause damage to the peripheral nervous system, mostly in immunocompromised patients.28
Most infective neuropathies tend to be asymmetrical in distribution rather than symmetric and have an acute onset corresponding
with a history of infection, however, HIV-derived neuropathies may present in a similar way to symmetric distal neuropathy
in people with diabetes, e.g. insidious, distal symmetrical with a limited history of infection.28
Systemic causes of peripheral neuropathy
In addition to diabetes, many other systemic metabolic, haematological and endocrine disorders, such as chronic liver
disease, alcoholism, renal failure, nutrient deficiencies, paraproteinaemic disorders and thyroid dysfunction can cause
peripheral neuropathy. Nerve damage in people with systemic disorders usually occurs due to impairment of nutrient transfer,
waste product removal or manufacture of necessary tissue products. Clinical and biochemical vitamin B12 deficiency is
common in people with diabetes,29 and metformin can reduce B12 absorption. It is still unclear, however, if
metformin treatment results in a clinically significant deficiency, as a peripheral neuropathy caused by vitamin B12 deficiency
is clinically indistinguishable from that caused by diabetes.29 Regardless of the mechanism an assessment
of vitamin B12 levels should form part of the work-up in a person with diabetes and peripheral neuropathy.
Other causes
Many medicines can cause or contribute to neuropathies, e.g. anti-infectives such as chloroquine, metronidazole and
nitrofurantoin; cardiovascular medicines, such as amiodarone; chemotherapy agents; colchicine; and phenytoin.12
In addition, neuropathy may sometimes be idiopathic (estimates are up to 20%, even in specialist centres), i.e. no identifiable
cause.
Inherited neuropathy
Hereditary neuropathies range from mild conditions with symptoms which arise in early adulthood to severe conditions,
present from birth or infancy, that cause significant disability. The most common inherited peripheral neuropathies are
a group of conditions known as Charcot-Marie-Tooth disease which arise from gene mutations that code for neuronal proteins
– primarily affecting the myelin sheath but also the axon. A positive family history, with an insidious onset, very gradual
progression, a lack of sensory symptoms despite clear sensory signs and the presence of pes cavus (high arched foot) suggest
an inherited process.