In this article 
View
/ Download pdf version of this article
Amenorrhoea
Amenorrhoea is the absence of menstruation flow. It can be classified as either primary or secondary,1 relative
to menarche:
- Primary amenorrhoea: absence of menses by age 16 years in a female with appropriate development of secondary sexual
characteristics; or absence of menses by age 13 years and no other pubertal maturation2
- Secondary amenorrhoea: lack of menses in a previously menstruating, non-pregnant female, for greater than six months2
Primary amenorrhoea
Key messages:
- The most common cause of primary amenorrhoea in a female with no secondary sexual characteristics is a constitutional
delay in growth and puberty. In the first instance, watchful waiting is the most appropriate course.
- For females with primary amenorrhoea but who have secondary sexual characteristics, pelvic ultrasound is indicated.
Evaluation of primary amenorrhoea
Causes of primary amenorrhoea should be evaluated in the context of the presence or absence of secondary sexual
characteristics.2
Absence of secondary sexual characteristics
The most common cause of primary amenorrhoea in a female with no secondary sexual characteristics is hypogonadotropic
(i.e. low LH and FSH) hypogonadism, often due to a constitutional delay in growth and puberty. A detailed family history
may reveal a familial aspect to this. If there is a positive family history, watchful waiting is appropriate in the first
instance, although the time will depend upon the age of the patient at presentation.
Testing LH and FSH is unhelpful in determining constitutional delay of puberty as low levels can be due to a delay but
also a number of other conditions (Figure 1).
Primary amenorrhoea in females with no secondary sexual characteristics may also be caused by hypergonadotropic (i.e.
high LH and FSH) hypogonadism. This is usually caused by premature ovarian failure, or gonadal dysgenesis – the
most common form of female gonadal dysgenesis is Turner syndrome (45X0 karyotype).
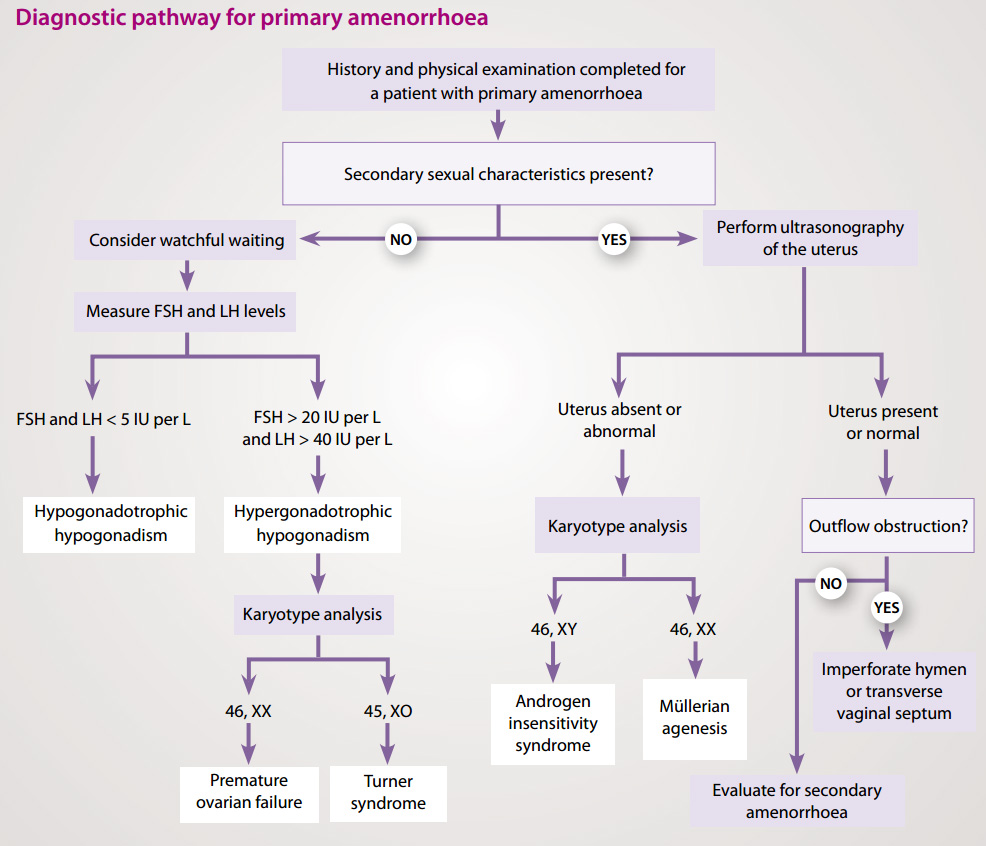
Figure 1: Investigations for primary amenorrhoea (adapted from Master-Hunter, 2006)3
Causes of primary amenorrhoea2
- Hypergonadotropic hypogonadism/primary hypogonadism/gonadal failure:
- Abnormal sex chromosomes e.g. Turner syndrome
- Normal sex chromosomes e.g. premature ovarian failure
- Hypogonadotropic hypogonadism/secondary hypogonadism:
- In many cases this may be due to a familial delay in puberty and growth. Other causes include congenital abnormalities
e.g. isolated GnRH deficiency, acquired lesions, endocrine disturbance, tumour, systemic illness or eating disorder.
- Eugonadism:
- Anatomic e.g. congenital absence of the uterus and vagina, intersex disorders or inappropriate endocrine feedback
mechanisms
In a large case series of primary amenorrhoea, the most common aetiologies were:2
- Chromosomal abnormalities causing gonadal dysgenesis (ovarian failure due to the premature depletion of all oocytes
and follicles) – 50%
- Hypothalamic hypogonadism including functional hypothalamic amenorrhoea – 20%
- Absence of the uterus, cervix and/or vagina, Müllerian agenesis – 15%
- Transverse vaginal septum or imperforate hymen – 5%
- Pituitary disease – 5%
Presence of secondary sexual characteristics
It is recommended that a female aged 16 years or over with secondary sexual characteristics, presenting with primary
amenorrhoea, is referred for a pelvic ultrasound to determine if the uterus is present. Absence of the uterus may be the
result of androgen insensitivity syndrome, where a person has a female external appearance despite a 46XY karyotype and
undescended testes. It can also be due to Müllerian ageneis which is a congenital malformation characterised by a
failure of the Müllerian ducts to develop, resulting in a missing uterus and variable malformations of the vagina.
If the uterus is normal, outflow tract obstruction should be considered. An imperforate hymen or a transverse vaginal
septum can cause outflow obstruction. This is most often associated with cyclic lower abdominal pain from blood accumulation
in the uterus and vagina. If there is no evidence of outflow obstruction, investigate as for secondary amenorrhoea (Table
1).
Secondary amenorrhoea
Secondary amenorrhoea is defined as a lack of menses for six months in a non-pregnant patient who was previously menstruating.
Key messages:
- Exclude pregnancy as it is the most common cause of secondary amenorrhoea
- Other than pregnancy , most cases of secondary amenorrhoea are caused by menopause, polycystic ovary syndrome (PCOS)
or functional anovulation e.g. excessive exercise, eating disorder, stress or certain medicines
- FSH, TSH and prolactin measurements will help identify most causes of secondary amenorrhoea. If there is suspicion
of pituitary disease, FT4 should also be tested.
Diagnostic evaluation of secondary amenorrhoea
Pregnancy should be first excluded by either urine pregnancy test or a serum hCG, rather than relying solely on history.
The history, physical examination and measurement of FSH, TSH and prolactin will help identify the most common causes
of amenorrhoea. In addition, for women with evidence of hyperandrogenism e.g. assessment of hirsutism and acne, and measurement
of testosterone would also be indicated.
Results can often be difficult to interpret (Table 1) therefore review or advice from a relevant specialist may be appropriate.
Table 1: Laboratory findings in common causes of secondary amenorhoea6
| Condition |
FSH |
Prolactin |
Testosterone |
Hyperprolactinaemia
|
Normal/low |
High |
Normal |
Polycystic Ovary Syndrome
|
Normal |
Normal/slightly increased in 5–30% |
Normal/moderately increased
Free androgen index increased |
Ovarian failure
|
High |
Normal |
Normal |
| Hypothalamic e.g. weight loss, excessive exercise, or stress |
Low/normal |
Normal |
Normal |
Causes of secondary amenorrhoea
After excluding pregnancy (and breast feeding), the most common causes of secondary amenorrhoea are:4,5
- Ovarian disease (40%) – ovarian failure due to normal or early menopause, hyperandrogenism e.g. PCOS, testosterone
supplementation
- Functional hypothalamic anovulation (35%) – due to excessive exercise, eating disorders, stress or some medicines
e.g. oral contraceptives, depot medroxyprogesterone
- Pituitary disease (19%) – has a similar presentation to functional hypothalamic amenorrhoea except for the
occasional additional finding of galactorrhoea in some women. Rare causes are sellar masses, other disease of the pituitary
and primary hypothyroidism.
- Uterine disease (5%) – Asherman’s syndrome is the only uterine cause of secondary amenorrhoea. This syndrome
results from acquired scarring of the endometrial lining, usually secondary to postpartum hemorrhage or endometrial
infection following instrumental procedure such as a dilatation and curettage.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is reported to affect between 5–10% of women of reproductive age.2,3
As well as taking a full history, examination of a woman with suspected PCOS should include an assessment of:
- Weight (both BMI and hip/waist ratio)
- Acne and hirsutism
- Blood pressure
Diagnostic criteria have been developed for PCOS
PCOS is a syndrome, so there is no single diagnostic test. There are several definitions of PCOS, but generally PCOS
is defined by the presence of hyperandrogenism (clinical or biochemical), ovarian dysfunction (oligo-anovulation or polycystic
ovaries), and the exclusion of related disorders.7 Table 2 compares diagnostic criteria for PCOS.
Investigation of PCOS
A clinical or biochemical finding of increased androgen levels along with either menstrual abnormalities or polycystic
ovaries on ultrasound will satisfy the current diagnostic criteria (Table 2). Other tests that should be considered when
investigating PCOS include FSH, TSH and prolactin levels.
Limited role of LH/FSH ratio8
In the past, the diagnosis of PCOS was often based upon the finding of an elevated ratio of LH to FSH in serum. Women
with PCOS tend to have elevated LH concentrations, with normal to low FSH. However, while some clinicians still test
LH and FSH, this is not required for a diagnosis of PCOS.
Once the diagnosis of PCOS is established, fasting glucose and lipids are recommended. For women with fasting glucose >5.5
mmol/L an oral glucose tolerance test (OGTT) is indicated. In addition, some clinicians recommend that OGTT should be
done for all women with PCOS who have a BMI > 30 and for all women aged over 40 years even if of normal weight.
 For more information about investigating PCOS see “Understanding
polycystic ovary syndrome” BPJ 12, April 2008.
For more information about investigating PCOS see “Understanding
polycystic ovary syndrome” BPJ 12, April 2008.
| Table 2: Diagnostic criteria for PCOS |
NIH criteria (1990)
|
Rotterdam Criteria (2003) |
AES Criteria (2006) |
All three of the following:
- Clinical or biochemical evidence of hyperandrogenism
- Oligomenorrhoea and/or anovulation
- Exclusion of other disorders
|
At least two of the following:
- Oligomenorrhoea and/or anovulation
- Clinical or biochemical signs of hyperandrogenism
- Polycystic ovaries
PCOS can be diagnosed only after the exclusion of related disorders (e.g. severe insulin resistance,
androgen-secreting neoplasms, Cushing’s syndrome, hyperprolactinaemia and thyroid abnormalities) |
All three of the following:
- Hyperandrogenism (clinical or biochemical)
- Ovarian dysfunction (oligomenorrhoea or anovulation and/or polycystic ovarian morphology)
- Exclusion of other androgen excess or related disorders
PCOS is predominantly a disorder of androgen excess |
The NIH Critera were developed first and therefore are most commonly used.
The Rotterdam criteria expanded the NIH definition.
The AES reviewed all available data and recommended an evidence-based definition. |
| NIH = National Institutes of Health, Rotterdam = European Society for Human Reproduction
and Embryology and the American Society for Reproductive Medicine, AES = Androgen Excess Society |
An overview of dysfunctional uterine bleeding
Any uterine bleeding which is outside the normal menstrual patterns can be termed as dysfunctional uterine bleeding.9 It
can be caused by a wide variety of local and systemic diseases or related to medicines e.g. hormonal contraceptives. However,
most cases of dysfunctional uterine bleeding are related to anovulatory cycles, menopause, pregnancy, structural uterine
pathology e.g. fibroids, polyps or adenomyosis or a disorder of haemostasis or neoplasia. Trauma and infection are less
common causes.
Laboratory studies for dysfunctional uterine bleeding
Investigations depend on individual presentation and examination.12 In many cases, it will be appropriate
to first exclude pregnancy and cervical or uterine cancer as causes of uterine bleeding.
A pregnancy test is indicated for women of reproductive age with dysfunctional uterine bleeding, to exclude intrauterine
or ectopic pregnancy, or gestational trophoblastic disease (hydatiform mole).
Any malignancy of the genital tract can cause dysfunctional bleeding. It can be difficult to determine whether bleeding
is from an endocervical or endometrial source, so cervical cancer must be excluded. Any visible cervical lesion should
be biopsied, even if cervical cytology is negative for malignancy.
Depending upon the history, clinical exam and initial evaluations, a second tier of laboratory testing may be appropriate.
This may include:
- Ultrasound examination and hysteroscopy – useful for further evaluating women whose findings
on pelvic examination are uncertain
- TSH – In women with signs and symptoms of hypo or hyper-thyroidism
- Coagulation tests – only in women with history suggestive of haemostatic defect e.g. frequent
nosebleeds, easy bruising
- Complete blood count (CBC) – in women with evidence of anaemia
- Chlamydia/gonorrhoea – if cervicititis, vaginal discharge and/or pelvic tenderness
- Prolactin – useful in women complaining of oligomenorrhoea, particularly with galactorrhoea
Terminology for dysfunctional uterine bleeding
There are a number of terms used to define abnormal frequency, duration or volume of uterine bleeding.10
Menorrhagia — prolonged (greater than seven days) or excessive ( > 80 mL daily) uterine bleeding
occurring at regular intervals
Oligomenorrhoea — regular bleeding that occurs at an interval greater than 35 days
Polymennorrhoea — regular bleeding that occurs at an interval less than 24 days
Metrorrhagia and menometrorrhagia – metrorrhagia refers to light bleeding at
irregular intervals. Menometrorrhagia refers to heavy bleeding at irregular intervals
Intermenstrual bleeding — bleeding that occurs between menses or between expected hormone withdrawal
bleeds in women using hormonal contraception or postmenopausal hormone therapy
Premenstrual spotting — light bleeding preceding regular menses
Post menopausal bleeding — recurrence of bleeding or spotting in a menopausal women at least
six months to one year after cessation of cycles
Postcoital bleeding — vaginal bleeding or spotting that is noted within 24 hours of vaginal
intercourse
Perimenopause and menopause
The average women begins menopause at approximately age 50 years. It is a retrospective diagnosis made following the
cessation of menses for 12 months or more. The menopausal transition begins with variations in menstrual cycle length
and ends with the final menstrual period. The perimenopausal period ends one year after the final menstrual period.11,12
Although hormone levels change throughout the menopausal period, hormone measurements are not useful for predicting
the stage of the transition, or the date of the final menstrual period. FSH levels can vary significantly across cycles,
so the use of FSH for predicting menopause in individual women is limited.11,12
In women aged over 45 years with symptoms suggestive of menopausal transition e.g. irregular menstrual cycles and menopausal
symptoms such as hot flushes, sleep disturbance, vaginal dryness or joint and muscle aches, no laboratory testing is required.11,12
In women aged under 45 years with symptoms suggestive of menopausal transition, consider the following investigations:
initially exclude pregnancy, if negative consider possible PCOS and measure prolactin, TSH, FSH levels and testosterone.11,12
Sexual dysfunction – loss of libido
Loss of libido has been reported to affect up to 40% of women at some time in their life.13
A full history and clinical examination, including sexual history and relationship factors is important. A key requirement
for the evaluation of female sexual dysfunction is to determine whether sexual issues are associated with personal stress.13
The presence of any serious medical condition is likely to impair sexual function not only because of the condition
itself, but also due to the associated impact on psychological well-being. In addition, poor self-assessed health is often
associated with sexual problems.13
Laboratory evaluation of sexual dysfunction
Laboratory testing should be performed only if indicated by history or examination.
Except in limited specialist settings, androgen levels are not helpful when evaluating the cause of a sexual problem.
The correlation between androgen levels and sexual dysfunction is considered weak, apart from a few well defined situations
such as proven pituitary or adrenal insufficiency or past bilateral oophorectomy. Similarly, testing oestradiol or other
hormones e.g. FSH and prolactin, has limited utility in evaluating sexual dysfunction.13
Acknowledgement
Thank you to Dr Anna Fenton, Endocrinologist, The Oxford Clinic, Christchurch for expert guidance
in developing this article.
References
- Assessment of secondary amenorrhoea. Best Practice. BMJ Publishing Group. Available from: http://bestpractice.bmj.com/best-practice/monograph/1102.html (Accessed
August, 2010).
- Welt C, Barbieri R. Etiology, diagnosis and treatment of primary amenorrhoea. UpToDate 2009. Available from: www.uptodate.com
(Accessed August, 2010).
- Master-Hunter T, Heiman D. Amenorrhoea: evaluation and treatment. Am Fam Physician. 2006;73(8):1374-82.
- Pinkerton JV. Amenorrhoea. The Merck Manuals. Available from: www.merck.com/mmpe/sec18/ch244/ch244b.html#CBBFFDAF (Accessed
August, 2010).
- Welt C, Barbieri R. Etiology, diagnosis and treatment of secondary amenorrhoea. UpToDate 2008. Available from: www.uptodate.com
(Accessed August, 2010).
- Clinical Knowledge Summaries (CKS). Amenorhoea. Available from: www.cks.nhs.uk/amenorrhoea (Accessed
August, 2010).
- Azziz R, Carmina E, Dewailly D, et al. Position statement: Criteria for defining polycystic ovary syndrome as a predominantly
hyperandrogenic syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab. 2006; 91:4237-45.
- Barbieri R. Treatment of hirsutism. UpToDate 2010. Available from: www.uptodate.com (Accessed
August, 2010).
- Emedicine. Dysfunctional uterine bleeding: differential diagnoses & workup. Feb 1, 2010. Available from: http://emedicine.medscape.com/article/795587-diagnosis (Accessed
August, 2010).
- Goodman A. Terminology and evaluation of abnormal uterine bleeding in premenopausal women. UpToDate 2009. Available
from www.uptodate.com (Accessed August, 2010).
- Casper R. Clinical manifestations and diagnosis of menopause. UpToDate 2009. Available from: www.uptodate.com (Accessed
August, 2010).
- Fertnstert J. The menopausal transition. Fertility Sterility 2008;90(Suppl 3):S61.
- Shifren J. Sexual dysfunction in women: Epidemiology, risk factors, and evaluation. UpToDate 2010. Available from: www.uptodate.com (Accessed
August 2010).