Growing use of azithromycin in New Zealand means that we are in danger of increasing bacterial resistance to macrolide antibiotics, as has been the case in other countries. Macrolides are particularly important in New Zealand given our high rates of pertussis and rheumatic fever. It is not too late to act; azithromycin should only be prescribed for specific indications to make sure it works when we need it the most.
There is also a Peer Group Discussion on this article
In this article 
View / Download pdf version of this article
Azithromycin is an effective antibiotic, but it’s use must be preserved
Azithromycin is a macrolide antibiotic with a broad spectrum of activity. While azithromycin has a number of indications,
infectious diseases experts recommend that in New Zealand, azithromycin only be used in the following situations:*
- First-line indications: pertussis in children, chlamydia, gonorrhoea (for treatment of presumed co-infection with
chlamydia), acute non-specific urethritis
- Second-line indications: pelvic inflammatory disease as an alternative to doxycycline when chlamydia is present, pertussis
in adults when erythromycin is unable to be tolerated
Internationally, particularly in the United States, azithromycin is a widely used antibiotic. This has led to rapidly
increasing levels of resistance among some common pathogens, e.g. Streptococcus pneumoniae.1 In December, 2012, PHARMAC
widened subsidised access to azithromycin to allow for the treatment of pertussis in children. While this has been beneficial
for managing the pertussis epidemic we need to remain cautious with the use of azithromycin to avoid an increase in macrolide
resistance in New Zealand, as has been the case overseas.
* bpacnz. Antibiotic choices for common infections. 2013. Available from:
www.bpac.org.nz
How does antimicrobial resistance occur?
All use of antibiotics contributes to resistance, but suboptimal use of antibiotics is the most important cause of the
emergence and spread of resistant organisms. Prescribing antibiotics when they are not indicated (e.g. for viral infections),
prescribing a broad spectrum antibiotic when a narrow spectrum option would be adequate and prescribing antibiotics at
an inappropriate dose or duration of treatment all result in increased resistance.
Azithromycin in particular is more likely to contribute to the development of resistance because of its long half-life
of approximately three days.2 This results in low (i.e. sub-inhibitory) concentrations of the drug at sites of microorganism
carriage for several days, which promotes the selection of resistant strains of bacteria. Nasopharyngeal carriage of macrolide-resistant
streptococci following treatment with azithromycin has been observed in a number of studies.3
Azithromycin use in the United States: A cautionary tale
Azithromycin is used much more extensively in the United States, for a wider range of infections, than in New Zealand.
Azithromycin is perceived to have potential advantages over other macrolides because it has fewer adverse gastrointestinal
effects, requires less frequent dosing (once a day), and it usually requires a shorter duration of treatment (e.g. five
days). For these reasons, azithromycin became the most commonly prescribed antibiotic in the United States in 2011.4 However,
resistance is increasingly of concern, with recent studies showing high rates of azithromycin resistance, particularly
in pneumococci. Currently 30 – 35 % of pneumococci in the United States are resistant to macrolides.5 Resistance
rates to macrolides began to increase sharply in the 1990s, coinciding with the introduction of clarithromycin in the
United States in 1991 and azithromycin in 1992. In the early 1990’s rates of macrolide resistance in pneumococci were
approximately 10%, however, by the early 2000’s resistance rates had reached 30%.5
International studies have linked increasing use of macrolides, particularly azithromycin, with the increasing prevalence
of resistance in Streptococcus pyogenes6 and in Streptococcus pneumoniae.7 While the direct
consequences
of increasing rates of macrolide resistance are difficult to quantify, there have been cases of breakthrough bacteraemia
in patients treated with macrolides who were subsequently found to be infected with macrolide-resistant strains of S.
pneumoniae.8 Other cases highlight problems with macrolide resistance in Streptococcus pyogenes, e.g. two
cases of children in the United States who developed rheumatic fever following treatment of streptococcal pharyngitis
with azithromycin. Macrolide resistance was proven in one case and presumed in the other.9
Azithromycin use in New Zealand: halting the surge
Concerns about increasing macrolide resistance were raised prior to the funding change in December, 2012, which widened
access to azithromycin. This change allowed for funded treatment of pertussis using a liquid formulation of azithromycin
suitable for children (as well as tablets). Previously, funding for azithromycin had been restricted to a maximum of two
500 mg tablets per prescription, for the treatment of infections due to Chlamydia trachomatis. Following consultation,
PHARMAC added a restriction of five days supply to the new azithromycin listing.
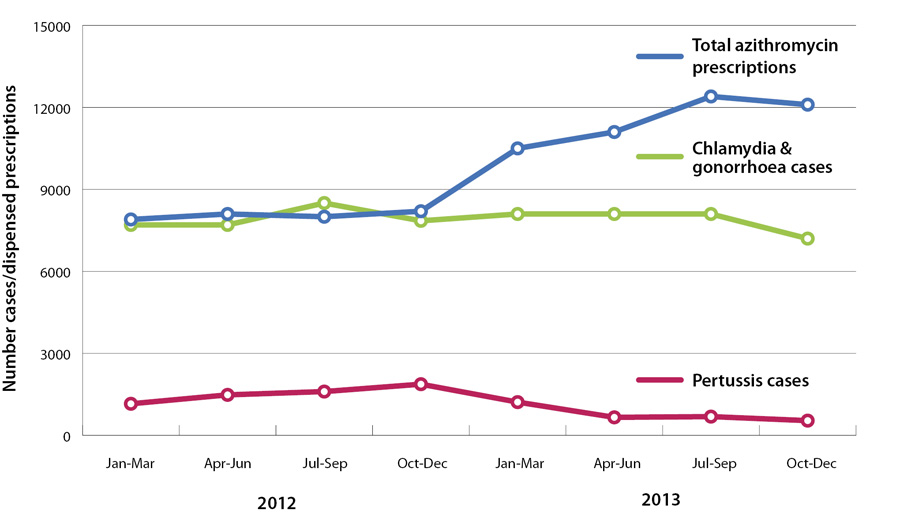
The number of azithromycin prescriptions in New Zealand has been increasing since the widening of access in December
2012 (Figure 1). While some increase may be expected due to the use of azithromycin for pertussis,
Figure 1 shows that pertussis rates began to decrease in early 2013, but dispensed azithromycin did not. Rates of chlamydia
and gonorrhoea also appear to be stable or decreasing.
ESR collects annual surveillance data on antimicrobial resistance rates in New Zealand. Data is not reported specifically
on azithromycin, but latest figures from 2012 show that 19.2% of S. pneumoniae and 3.9% of S. Pyogenes were resistant
to erythromycin.13
The final word
Unlike some other countries where macrolide resistance is already out of control, it is not too late to preserve the
effectiveness of macrolides in New Zealand. By prescribing azithromycin only for the conditions it is recommended for
(i.e. first-line antibiotic treatment for pertussis in children and first-line treatment for chlamydia, and second-line
antibiotic treatment for pelvic inflammatory disease) we can ensure that macrolide antibiotics remain effective when they
are needed the most. Wise use of antibiotics means prescribing the right antibiotic, for the right indication, to the
right person.
Figure 1:
Number of dispensed azithromycin prescriptions and number of cases of pertussis and gonorrhoea and Chlamydia (combined), 2012 – 2013.
References
- Jenkins SG, Farrell DJ. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis 2009;15:1260–4.
- Brayfield A (ed). Azithromycin. In: Martindale: The complete drug reference (online). London: Pharmaceutical Press
2014. Available from: www.medicinescomplete.com (Accessed
Apr, 2014).
- Malhotra-Kumar S, Lammens C, Coenen S, et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage
of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet
2007;369:482–90.
- Canavan N. Opposition growing against azithromycin for infections. Medscape, February 2014 Available from:
www.medscape.com (Accessed
Apr, 2014).
- Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory
airway diseases. Lancet Respir Med 2013;1:262–74.
- Gagliotti C, Nobilio L, Milandri M, et al. Macrolide prescriptions and erythromycin resistance of Streptococcus pyogenes.
Clin Infect Dis 2006;42:1153–6.
- Bergman M, Huikko S, Huovinen P, et al. Macrolide and azithromycin use are linked to increased macrolide resistance
in Streptococcus pneumoniae. Antimicrob Agents Chemother 2006;50:3646–50.
- Lonks JR, Garau J, Gomez L, et al. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant
Streptococcus pneumoniae. Clin Infect Dis 2002;35:556–64.
- Logan LK, McAuley JB, Shulman ST. Macrolide treatment failure in streptococcal pharyngitis resulting in acute rheumatic
fever. Pediatrics 2012;129:e798–802. doi:10.1542/peds.2011-1198
- U.S. Food and Drug Administration (FDA). Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart
rhythms. FDA Drug Safety Communication, 2013. Available from:
www.fda.gov/drugs/drugsafety/ucm341822.htm (Accessed
Apr, 2014).
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90.
- Mossholder A, Matthew J, Alexander J, et al. Cardiovascular risks with azithromycin and other antibacterial drugs.
N Eng J Med 2013;368:1665–8.
- Environmental Science and Research (ESR). Antimicrobial resistance data from hospital and community laboratories.
ESR, 2012. Available from:
https://surv.esr.cri.nz/antimicrobial/general_antimicrobial_susceptibility.php (Accessed,
Apr, 2014).