Key practice points:
- Many patients with atrial fibrillation (AF) are asymptomatic. Opportunistic assessment in primary care via pulse palpation is recommended in people aged ≥ 65 years, or earlier if they have an increased risk of AF, e.g. Māori or Pacific peoples, previous transient ischaemic attack (TIA), hypertension. If an abnormality is detected, AF can be confirmed via ECG.
- Oral anticoagulation alongside rate control is the key to managing most stable patients with newly diagnosed AF (in addition to consistent follow-up)
- The need for anticoagulation can be calculated using the CHA2DS2-VASc score; females with a score of ≥ 2 and males with a score of ≥ 1 are likely to benefit from an anticoagulant to reduce their risk of stroke. The decision to initiate treatment should also consider bleeding risk which can be assessed with tools such as HAS-BLED.
- Direct oral anticoagulants (DOACs) are now preferred over warfarin for stroke prevention, unless contraindicated:
- Dabigatran or rivaroxaban are both reasonable first choices with a similar clinical effect. Patient-specific features may dictate selection, e.g. dabigatran may be favourable in patients with a higher bleeding risk due to the availability of a reversal agent, whereas rivaroxaban may be preferable in those who prefer once daily dosing, have moderate renal dysfunction, or a history of dyspepsia.
- Aspirin or other antiplatelets are not recommended for long-term stroke prevention in patients with AF as they are less effective than anticoagulants
- Rate control is often preferred over rhythm control strategies for managing patients in primary care with AF symptoms:
- A beta blocker is generally first-line, but medicine choice is influenced by co-morbidities and should ideally be refined based on echocardiogram findings
- Target a heart rate < 110 bpm in most cases
- Cardiologist input should guide the use of rhythm control strategies in patients with symptomatic AF not responding to rate control; these include both pharmacological and electrical cardioversion, as well as more advanced procedures, e.g. catheter or surgical ablation
- Lifestyle modifications and optimising the management of co-morbidities is always essential to help limit AF symptom burden and the risk of long-term complications
This is a revision of a previously published article. What’s new for this update:
- A general article revision
- Emphasis on an individualised and progressive approach to treatment
- Discussion around patients who may be candidates for early rhythm control
- Comment on the role of smart watches in detecting AF
- Review of evidence regarding AF in athletes
Atrial fibrillation (AF) is the most common form of sustained cardiac arrhythmia in adults.1 In New Zealand, AF is reported to affect at least 5% of people aged 65 to 74 years and up to 11% of people aged over 75 years.2
The irregular cardiac rhythm associated with AF decreases the heart’s ability to efficiently pump blood and promotes clot formation. People with AF have a higher risk of mortality compared to those without AF, as well as a 2.5-fold increased risk of stroke, a five-fold increased risk of heart failure and 1.5-fold increased risks of myocardial infarction and dementia.3 AF is diagnosed in Māori and Pacific peoples on average nine years earlier than Europeans (66 years versus 75 years) and these groups have an even higher risk of stroke.2 Early detection in primary care is therefore essential to facilitate timely and effective management. However, even successful treatment does not completely reverse the increased risks associated with AF.
Consider opportunistic assessment of patients aged ≥ 65 years
A key barrier to identifying patients with AF in general practice is that they are often asymptomatic.1 If symptoms are present, the range and severity (e.g. palpitations, dizziness, shortness of breath [particularly on exertion], angina, fatigue), and extent of changes in heart rate and rhythm at diagnosis can vary widely.1 Opportunistic assessment for cardiac arrhythmia via radial pulse palpation during routine appointments is therefore an important strategy to detect AF in patients aged ≥ 65 years.4 If AF is suspected after radial pulse palpation, chest auscultation to evaluate apical pulse can strengthen diagnostic accuracy. Assessment is warranted at an earlier age for Māori and Pacific peoples or other groups with an elevated stroke risk, e.g. people with previous transient ischaemic attack or receiving pharmacological treatment for hypertension.1
ECG is required to confirm AF
If an arrhythmia is detected via pulse palpation, AF should subsequently be investigated and confirmed with an ECG at the appointment if the practice has a monitor available.1, 4 The typical pattern of AF on an ECG involves irregularly irregular RR intervals and no discernible, distinct P waves.1, 4 Small irregular fibrillatory waves are often observable between QRS complexes. For diagnostic purposes, AF is defined as lasting at least 30 seconds.1, 4 However, a standard 12-lead ECG only provides ten seconds of recording, so the presence of a typical AF pattern for the entirety of this period is sufficient to confirm AF in primary care.
In some cases, assessment with Holter monitoring may be required if the patient reports intermittent palpitations (i.e. paroxysmal AF is suspected; see below), however, detection may be ineffective if symptoms are less frequent or sporadic.5
Classifications of AF include:1, 4
- First diagnosed – AF has not been diagnosed before, irrespective of symptom presence or severity
- Paroxysmal – AF that resolves spontaneously or is cardioverted within seven days
- Persistent – continuous AF for more than seven days that has not resolved spontaneously; this includes episodes that are cardioverted after seven or more days
- Long-standing persistent – AF has lasted continuously for at least one year and attempts to restore or maintain sinus rhythm are still being considered or attempted
- Permanent – AF has been present for more than one year and cardioversion has been unsuccessful or has not been attempted. This applies when a joint decision has been made by the clinician and patient to accept the presence of AF and to not attempt further strategies to restore or maintain sinus rhythm. If further cardioversion attempts are made, the patient is re-classified as having long-standing persistent AF.
Proposed classifications for AF have changed over time
Conventionally, classification systems have focused on arrhythmia duration and emphasise interventions for existing AF (as above). In 2023, guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) suggested a new framework that acknowledges AF as a continuum of disease, and emphasises action to be taken for patients considered “at risk”.3
The 2023 ACC/AHA classification system includes:3
- Stage 1 (at risk for AF) – presence of modifiable risk factors
- Stage 2 (pre-AF) – evidence of structural or electrical findings further predisposing a patient to AF, e.g. atrial enlargement, short bursts of tachycardia, atrial flutter
- Stage 3 (AF) – patients may transition among different substages of AF, including paroxysmal, persistent and long-standing persistent, as well as a new designation “Successful AF ablation” (AF not present after percutaneous or surgical intervention)
- Stage 4 (permanent AF) – no further attempts at rhythm control (as above)
When considered from a primary care perspective, this framework serves as a reminder about the importance of addressing modifiable risk factors across all AF stages, including in people without established disease who are at increased risk as this may prevent onset, progression and limit adverse outcomes (see: “Address modifiable risk factors and co-morbidities”).3 In addition, the guidelines note that people with “pre-AF” could be considered candidates for more regular monitoring.3
Does the patient need to be referred for immediate cardioversion?
In a general practice setting, many patients with AF will be detected opportunistically, however some may be identified after presenting with symptoms, e.g. palpitations. Conventionally, after ECG confirmation, many patients would have been referred immediately for acute electrical cardioversion if symptom onset was within 48 hours, in an attempt to restore normal sinus rhythm and to avoid the need for long-term anticoagulation. However, evidence suggests this approach puts patients who are otherwise haemodynamically stable at unnecessary risk, and the timeframe for taking such action may be shorter than previously thought.
A 2017 study found that cardioversion more than 12 hours after symptom onset significantly increases the risk of thromboembolic complications.6 Most notably, for females aged 65 – 75 years without prior anticoagulation, 3.5% experience thromboembolic complications with electrical cardioversion performed between 12 – 48 hours after AF symptom onset, compared with only 0.4% when performed within 12 hours.6
Practice point:
If symptom onset is > 12 hours ago, emergency referral is not usually required
Unless the onset of AF is definitely less than 12 hours, referral for electrical cardioversion is not recommended in haemodynamically stable patients without prior anticoagulation due to the risk of thromboembolic complications. Many patients with newly identified AF spontaneously convert to sinus rhythm within the next few days after onset, and use of a rate control medicine and assessment for anticoagulation suitability is usually a safer strategy. These medicines may alleviate the need for cardioversion at a later time (either pharmacological or electrical).
Haemodynamically unstable patients require immediate referral
Patients with signs of haemodynamic instability (e.g. hypotension, peripheral cyanosis, ongoing chest pain suggestive of myocardial ischaemia) should be referred for acute cardiology assessment.1, 3, 4 In these situations, the associated instability is rarely directly due to AF, and a comprehensive review is required to exclude secondary causes such as infection or heart failure.
The role of smart watches in detecting AF

Personal wearable devices, such as smart watches, are available that contain optical sensors that intermittently process pulse rate data and use custom algorithms to identify episodes suggestive of AF. Clinicians may have patients request appointments to follow-up on alerts from their device, or use it as a discussion point with patients, e.g. do you know what your resting pulse is?
The positive predictive values for AF reported in studies involving such devices range from 84 – 98%,6, 7 suggesting that these alerts are of clinical significance. However, this does not replace the need for usual diagnostic investigations for AF, i.e. pulse palpation and ECG.3 If an abnormality is not detected via subsequent pulse palpation, performing an ECG may still be a reasonable action. If this ECG does not confirm AF, Holter monitoring may be considered to encompass the possibility of intermittent episodes (i.e. paroxysmal AF).
Investigations into the potential use of smart watches, apps or similar devices for long-term AF monitoring and management are ongoing. These devices may be useful for tracking heart rate and rhythm outside of clinical appointments, however, a number of factors may influence the accuracy of measurements, including positioning, interference and sensor quality/characteristics.8
Assuming referral for electrical cardioversion is not indicated, an individualised and progressive approach to AF management is recommended in general practice in haemodynamically stable patients (Figure 1). Management decisions are informed via comprehensive baseline assessment, as patient-specific features influence the suitability of medicines and interventions. Regardless of the approach taken, optimising the management of modifiable risk factors (and co-morbidities) should be a key component of treatment (see: “Address modifiable risk factors and co-morbidities”), in addition to addressing stroke risk (see: “Managing stroke risk with anticoagulants”) and symptom management (see: “Managing symptoms with rate and rhythm control strategies”).3
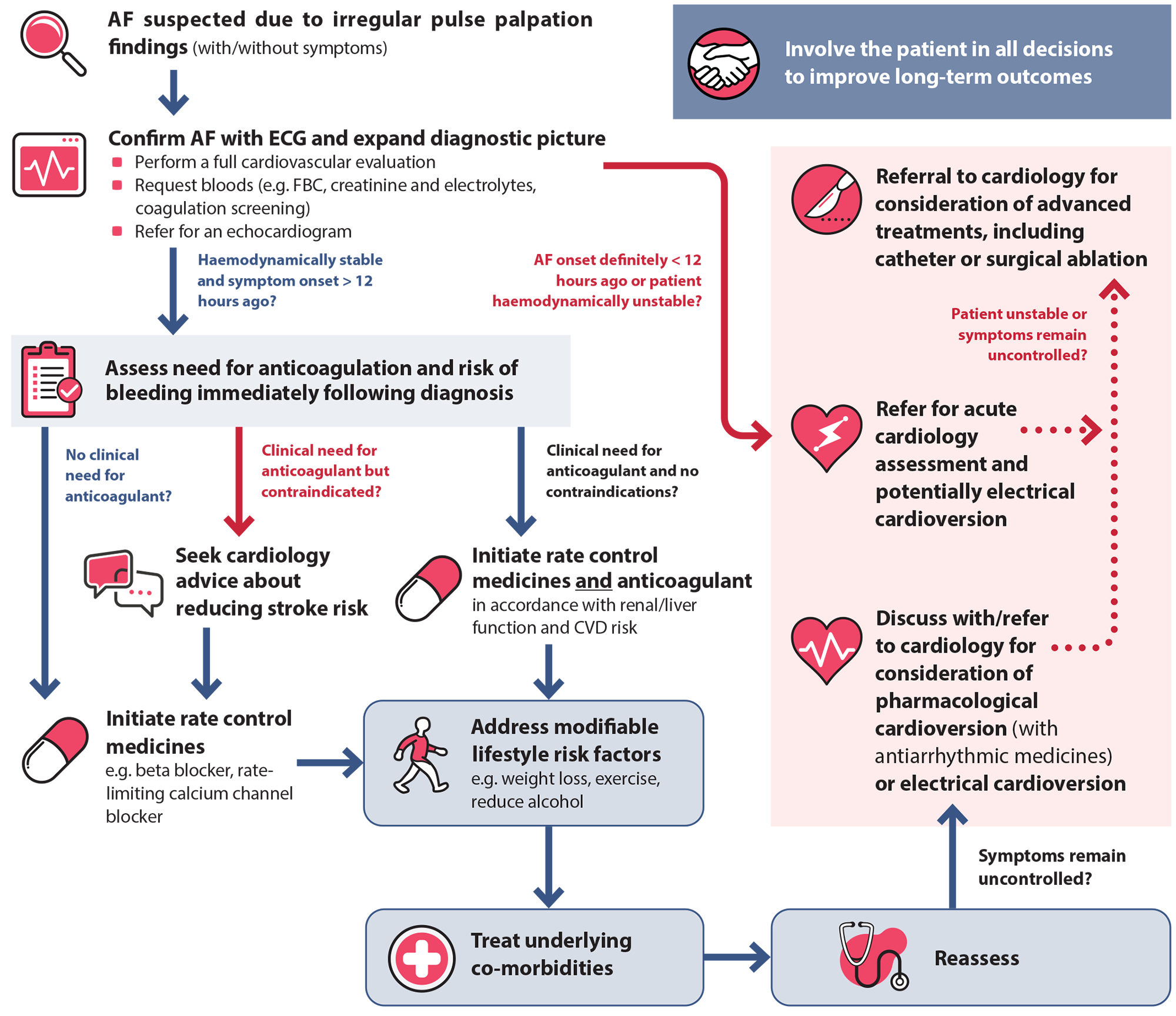
Figure 1. An individualised and progressive approach to AF management in primary care.
AF = atrial fibrillation; CVD = cardiovascular disease; ECG = electrocardiogram
Expand the diagnostic picture
- Perform a full cardiovascular assessment,4 including history (e.g. onset, frequency, duration and severity of any associated symptoms, CVD risk factors), clinical examination (e.g. blood pressure, looking for signs of heart, lung and thyroid disease) and assessment of co-morbidities. Consider if the patient has a reversible underlying non-cardiac condition (such as pulmonary embolism, hyperthyroidism or excessive alcohol consumption) causing their symptoms and changes in heart rate, or clinical evidence of a cardiac condition that may have contributed to the development of AF, e.g. myocardial ischaemia.
- Request laboratory tests,9 including FBC, creatinine and electrolytes, coagulation screening (e.g. INR, APTT) if oral anticoagulants will likely be used, as well as LFTs and thyroid function tests if not requested recently. N.B. Many primary care clinicians may not currently request a coagulation screen before starting a patient on an anticoagulant, but this is regarded as best practice to rule out pre-existing clotting disorders and to establish a baseline (if warfarin is ultimately used).
- Refer for an echocardiogram but do not delay treatment.4 The results from transthoracic echocardiography may influence long-term treatment selection. However, access can be variable and immediate action is generally prioritised according to history and examination findings. In some regions, general practitioners may be able to refer patients directly; in others, referral to a cardiologist may be required. Check local HealthPathways for guidance.
Assess the patient’s stroke and bleeding risk
The stroke risk associated with AF is comparable regardless of the underlying pattern and whether or not symptoms are present.1 Patients with AF typically experience strokes that are more severe than those occurring due to other causes.10 The need for anticoagulant treatment to reduce this risk should therefore be considered immediately following diagnosis (see: “Managing stroke risk with anticoagulants”). In primary care, this decision can be guided by balancing the patient’s CHA2DS2-VASc score (stroke risk) against their HAS-BLED score (bleeding risk).4
N.B. Other stroke and bleeding risk assessment tools are also available and may be used alongside or potentially replace the CHA2DS2-VASc/HAS-BLED tools in the future (see: “New risk assessment tools are being considered in guidelines”).
For online CHA2DS2-VASc and HAS-BLED calculators, see: www.chadsvasc.org
CHA2DS2-VASc scoring
International guidelines generally recommend using the CHA2DS2-VASc score to estimate stroke risk in patients with AF as it has a higher sensitivity than other tools (available at the time for comparison).1, 3–5 AnticoagulatioThe Cockcroft-Gault equation n should generally be considered for females with a CHA2DS2-VASc score ≥ 2 in and males with a score ≥ 1 (Table 1).1, 3 Females with a CHA2DS2-VASc score of 1 and males with a score of 0 should not be offered anticoagulants as their risk of stroke is low, with rates of ischaemic stroke less than 1 per 100 people per year;11, 12 the benefit from anticoagulant use is unlikely to outweigh the risks of treatment. In such cases, the management should focus on addressing modifiable risk factors and optimising the treatment of co-morbidities (see: “Address modifiable risk factors and co-morbidities”). Antiplatelet treatment is not recommended as an alternative to anticoagulation in these patients.1
Table 1. CHA2DS2-VASc scoring to predict stroke risk in patients with AF.1
|
Risk factor for stroke |
Points |
C |
Congestive heart failure or left ventricular systolic dysfunction |
1 |
H |
Hypertension or current antihypertensive medicine use |
1 |
A2 |
Aged 75 years or over |
2 |
D |
Diabetes mellitus |
1 |
S2 |
Stroke, transient ischaemic attack or thromboembolism |
2 |
V |
Vascular disease (e.g. myocardial infarction) |
1 |
A |
Aged 65 – 75 years |
1 |
Sc |
Sex category – female |
1 |
|
Total (0 –9) |
|
|
Consider offering anticoagulation to patients with the following scores depending on their bleeding risk (Table 2) |
≥ 1 for males
≥ 2 for females |
HAS-BLED scoring
Patients with increased stroke risk are also likely to be at greater risk of experiencing a major bleed with anticoagulant use as the associated risk factors largely overlap, e.g. age is a risk factor for both ischaemic stroke and bleeding in patients with AF.4, 13
HAS-BLED scoring can help identify bleeding risk factors and guide management decisions (Table 2).1 While the risk of bleeding increases with higher scores, there are no specific cut-offs to identify patients who should not be initiated on an anticoagulant, particularly as the consequences of a stroke are typically more severe than the consequences of a bleed.1, 3
The need for anticoagulation should therefore be primarily decided by the CHA2DS2-VASc score, and the HAS-BLED score used to:1, 3
- Consider the balance of benefits and risks of anticoagulant treatment, e.g. for patients with high HAS-BLED scores and low CHA2DS2-VASc scores (≤ 2) the risk of anticoagulation may outweigh benefits
- Identify factors which could potentially be altered to reduce a patient’s bleeding risk, e.g. uncontrolled hypertension, NSAID use, high alcohol intake
- Identify patients at higher risk of bleeding who could benefit from more frequent follow-up or intensive management
Table 2. HAS-BLED scoring to predict bleeding risk in patients with AF.1
|
Risk factor for bleeding |
Score |
H |
Hypertension (systolic blood pressure > 160 mmHg or uncontrolled blood pressure) |
1 |
A |
Abnormal renal and/or liver function, e.g. liver disease or aminotransferase levels > 3 times the upper limit of normal |
1 point for each |
S |
Previous history of stroke |
1 |
B |
Bleeding (prior major bleeding or predisposition to bleeding, e.g. anaemia) |
1 |
L |
Labile INR (unstable/high or time in therapeutic range < 60%) |
1 |
E |
Elderly (aged > 65 years) or extreme frailty |
1 |
D |
Drugs or alcohol use (criteria includes patients who drink ≥ 8 standard drinks per week and/or medicine use predisposing to bleeding, e.g. antiplatelet medicines or NSAIDs) |
1 point for each |
|
Total (0 – 9) |
|
Oral anticoagulants are first-line for stroke prevention in patients with AF; they reduce both stroke risk and mortality, with greater benefits expected in patients at higher risk.11 Despite evidence suggesting that 94% of patients with AF in primary care in New Zealand should be considered for anticoagulant treatment (based on CHA2DS2-VASc scoring), much fewer are actually prescribed an anticoagulant. A study found that in 2014, only around 60% of people at high risk of stroke were prescribed an anticoagulant in New Zealand primary care.2 On average, Māori were more likely to be prescribed anticoagulant treatment compared with Europeans, irrespective of their stroke risk level.2
Absolute contraindications to oral anticoagulant use includes:1
- Active serious bleeding
- Bleeding-associated co-morbidities, e.g. severe thrombocytopenia (< 50 platelets/microlitre) or severe anaemia
- History of recent high-risk bleeding event, e.g. intracranial haemorrhage (N.B. Patients with recent intracranial haemorrhage may still benefit from oral anticoagulant use, however, these decisions should be made in secondary care following neuroimaging)
Direct oral anticoagulants (DOACs) generally recommended over warfarin
Direct oral anticoagulants (DOACs) are typically preferred over warfarin as they are superior for reducing the risk of stroke and all-cause mortality, reduce the risk of intracranial bleeding and have a comparable risk of major bleeding.1, 16 DOACs also have more predictable pharmacokinetic/pharmacodynamic properties (no INR monitoring required), fewer medicine and food interactions and a more rapid onset of action compared with warfarin.17, 18
For further information on DOAC (and warfarin) use in primary care, including factors to consider when selecting between different options, as well as dosing and monitoring information, see: bpac.org.nz/2023/anticoagulants.aspx
Recommended doses for DOACs in patients with AF
The funded DOACs in New Zealand, dabigatran and rivaroxaban, have a similar clinical effect and are both suitable first-line options:
- Dabigatran – 150 mg, twice daily if CrCl* > 50 mL/min or 110 mg, twice daily, for patients aged ≥ 80 years (because of the likelihood of an age-related decline in renal function), patients aged 75 – 80 years with low thromboembolic risk and high bleeding risk, or patients with a CrCl 30 – 49 mL/min (contraindicated if CrCl < 30 mL/min)
- Rivaroxaban – 20 mg, once daily if CrCl > 50 mL/min or 15 mg, once daily in patients with a CrCl 15 – 49 mL/min (contraindicated if CrCl < 15 mL/min)
*The Cockcroft-Gault equation is used to calculate creatinine clearance (in mL/min) and guide DOAC dosing in preference to the estimated glomerular filtration rate (eGFR – mL/min/1.73 m2).1 Dabigatran is contraindicated at a higher CrCl threshold compared with other DOACs as it undergoes more significant renal excretion.
Warfarin still preferrable in some situations
Use of warfarin has decreased significantly since the introduction of DOACs. However, there are some situations in which DOACs are contraindicated (e.g. mechanical heart valves) or there is insufficient evidence to support their use (e.g. moderate-to-severe mitral stenosis, severe liver or renal dysfunction), and warfarin should be used instead.1, 4, 19 Other reasons for considering warfarin include patients with a significant history of gastrointestinal disease or co-morbid antiphospholipid syndrome (rare) or patients who develop thrombosis while taking a DOAC.
Anticoagulation during pregnancy
Anticoagulation decisions in patients who are pregnant should usually be made under the guidance of an obstetrician, with use typically being reserved for those at high risk. If required, anticoagulation with a low molecular weight heparin such as enoxaparin is generally considered the best approach during pregnancy when managing AF as it does not cross the placenta or cause fetal anticoagulation.1, 18 There is insufficient evidence to support the safety of DOAC use during pregnancy, and warfarin is also generally avoided (but is sometimes considered at certain stages after the first trimester in patients at high risk under specialist guidance).
Antiplatelet medicines alone are no longer recommended for long-term stroke prevention in patients with AF
Oral anticoagulants are superior to aspirin and/or clopidogrel for the prevention of stroke, systemic embolism and myocardial infarction in patients with AF,1, 4 and are associated with a lower risk of major bleeding and intracranial haemorrhage.20 Long-term antiplatelet medicine use alone is therefore no longer recommended in patients with AF, even if they are at very low risk of stroke (i.e. CHA2DS2-VASc score of 1 for females or 0 for males).1, 3
After an acute coronary syndrome (ACS) or coronary stent procedure, patients with AF will likely have antiplatelet medicines initiated in secondary care. The risk of bleeding is increased with concurrent use of antiplatelets and anticoagulants,1 and prescribers in primary care should confirm the intended duration of treatment with the antiplatelet before renewing the prescription. If required, clopidogrel is the recommended antiplatelet for most patients when combined with oral anticoagulants, however low-dose aspirin is sometimes used (e.g. 100 mg).21 Triple antithrombotic treatment (i.e. dual antiplatelet treatment with aspirin and clopidogrel plus an oral anticoagulant) is generally avoided; if needed it should only be taken for a short duration, e.g. ≤ 30 days.21
Withholding or stopping oral anticoagulant use
- Bleeding risk. An elevated bleeding risk alone does not automatically make patients ineligible for oral anticoagulant use.1 For further information, see: “HAS-BLED scoring”.
- Older age. Do not withhold oral anticoagulants in patients at high risk of stroke based on concerns around their age or falls risk.6 The benefits of using oral anticoagulants generally outweigh the risk of complications.1 Polypharmacy can be an issue in older patients with multiple co-morbidities; integrated AF management strategies should consider potential medicine interactions, and medicine choices or doses may need to be adapted to reduce the risk of complications.
- Discontinuation.The decision to stop anticoagulant medicines should be based on a continued evaluation of the patient’s stroke and bleeding risk (e.g. determined by CHA2DS2-VASc and HAS-BLED scores) and not because AF has reverted to sinus rhythm or symptom resolution.5
After performing a cardiovascular assessment and considering the need for anticoagulants, the next step is to address AF symptoms. There are two main approaches:1, 3–5
- Rate control – aims to improve symptoms by reducing heart rate
- Rhythm control – attempts to restore sinus rhythm using either electrical cardioversion or pharmacological cardioversion with antiarrhythmic medicines
Rate control is usually the initial step in primary care. Randomised controlled trial evidence suggests both strategies in patients with AF have similar effects on quality of life and clinical outcomes, including mortality risk.1, 4, 22 In primary care, rate control alone is the preferred initial symptomatic treatment as the medicine regimens are simpler and there are generally fewer associated adverse effects.1, 4, 5
Early rhythm control may still be an appropriate initial strategy in select cases.22 This decision should usually be made with cardiology advice, and may include patients:1, 3–5
- Who are younger, particularly those who are highly physically active and without co-morbidities
- With a high initial symptom burden
- With symptomatic paroxysmal attacks
- With a clear trigger or reversible cause for AF, e.g. recent cardiac surgery, acute illness (e.g. pneumonia), binge drinking
- With heart failure primarily caused or exacerbated by AF
Switching from rate control to rhythm control. Patients with ongoing symptoms despite use of rate control medicines may benefit from a rhythm control strategy.1, 4, 5 There is no consensus on when this transition to rhythm control should take place in primary care; it will depend on patient characteristics, their symptoms and preferences. In practice, it may be reasonable to trial rate control for a few months prior to considering rhythm control. However, a longer duration of persistent AF reduces the probability of successful subsequent rhythm control (e.g. due to progressive atrial remodelling); this needs to be considered during follow-up reviews. Clinicians should discuss any patients being considered for rhythm control with a cardiologist to guide treatment decisions. N.B. Rate and rhythm control strategies can be used in combination in some patients.1, 23
There is ongoing debate in the literature as to whether a rate or rhythm control strategy is preferable, particularly in patients with recent onset AF. In New Zealand primary care, early echocardiogram and discussion with a cardiologist about starting rhythm control would be warranted in younger or more symptomatic patients. For a review article discussing early rhythm control, see: www.jacc.org/doi/10.1016/j.jacc.2022.03.337.
Rate control: Beta blockers are often a good first choice
Both beta blockers and rate-limiting calcium channel blockers (e.g. diltiazem, verapamil) are suitable first-line options for rate control in patients with AF (Table 3).1, 3–5 The beta blocker sotalol should not be prescribed for rate control in patients with AF because it has the potential to cause arrhythmias (it can be used for rhythm control, if required).4
The choice between a beta blocker or rate-limiting calcium channel blocker can usually be made based on the patient’s symptoms, heart rate, co-morbidities and any adverse effects.1, 5 Treatment selection should ideally be informed based on echocardiogram results assessing cardiac structure and function,4 however, access varies significantly across New Zealand. In many cases, prescribing a beta blocker is a good first choice in the absence of echocardiography findings, as verapamil and diltiazem are not recommended in patients with left ventricular ejection fraction (LVEF) < 40% or heart failure with reduced ejection fraction (HFrEF) due to negative inotropic effects.3, 4 The initial dose of a beta blocker or calcium channel blocker can be determined based on the degree of elevation of the patient’s heart rate and other characteristics, e.g. LVEF.1
For further information on choosing an appropriate beta-blocker for patients with co-morbidities, see: “Beta blockers for cardiovascular conditions”, available from bpac.org.nz/2017/beta-blockers.aspx
Table 3. Recommended medicines for rate control in patients with AF.1, 4, 18
| Clinical condition |
Monotherapy |
Combination treatment |
LVEF ≥ 40% |
Beta blocker: (first-line)
- Bisoprolol* – 1.25 – 20 mg, once daily (usual range 2.5 mg – 10 mg)
- Metoprolol succinate – 23.75 – 190 mg, once daily†
- Carvedilol* – 3.125 – 50 mg, twice daily
|
Add digoxin to either the beta blocker or rate-limiting calcium channel blocker
Or
Combine a beta blocker with diltiazem (i.e. without digoxin)
N.B. Use this combination with caution, especially in patients with LVEF 40 – 49% or cardiac conduction abnormalities, as the effects can be difficult to predict. Do not use verapamil with a beta blocker due to the risk of hypotension and systole as a result of the potentially additive negative inotropic effects. |
Rate-limiting calcium channel blocker: (first-line)
- Diltiazem* – 60 mg, three times daily, increased to 360 mg maximum total daily dose in divided doses (immediate-release form) or 120 – 360 mg, once daily (modified-release form)
- Verapamil – 40 – 120 mg, three times daily (immediate-release form) or 120 – 240 mg, once daily, increased to 240 mg, twice daily, if necessary (modified-release form*)
|
Digoxin** – 0.75 – 1.5 mg, over 24 hours in divided doses (loading dose) then 0.0625 – 0.25 mg, once daily (maintenance dose) |
Add beta blocker, diltiazem or verapamil |
Signs of congestive heart failure and LVEF < 40% |
Beta blocker (first-line) – options and dosing as above |
Add digoxin |
Digoxin**– dosing as above |
Add beta blocker at lowest possible dose for acute heart rate control |
Haemodynamic instability or severely reduced LVEF |
Amiodarone – rarely initiated for rate control in primary care unless under cardiology advice |
Add digoxin |
*Unapproved indication
† Recommend dose range differs from that listed on the NZ Formulary (NZF) for general “arrhythmias”, which lists the lower threshold as 95 mg, once daily. Smaller doses, e.g. 23.75 mg, can be a suitable starting point for select patients with AF as they are still often effective and reduce the risk of adverse effects.
** Used infrequently for monotherapy in primary care due to its potential for medicine interactions (see the NZF interactions checker: www.nzf.org.nz), lack of effect on heart rate during physical activity and narrow therapeutic index. Sometimes considered for initial rate control in patients with non-paroxysmal AF if they lead a sedentary lifestyle (or if other options are contraindicated). Monitoring of digoxin serum concentrations (at least six hours after last dose) may be necessary to optimise treatment and reduce the risk of adverse effects.
AF = atrial fibrillation; LVEF = left ventricular ejection fraction
Treatment targets
Most patients will benefit from targeting an initial resting heart rate of < 110 bpm.1, 3 A more intensive approach aiming for a greater reduction in heart rate, e.g. < 80 – 90 bpm, is appropriate for patients with ongoing symptoms or who have known left ventricular dysfunction.1 There are no specific follow-up recommendations for patients initiated on rate control medicines as this will largely depend on the degree of heart rate elevation and medicine selection and co-morbidities (see: “Follow-up and referral of patients with AF managed in primary care”). For patients with a low to moderate heart rate elevation, consider starting at a low dose (Table 3) and reviewing after one to two weeks to assess treatment effect before deciding whether further titration is required.
Intensifying treatment
Combination treatment can be considered if the patient’s heart rate target is not achieved with optimal monotherapy doses (Table 3).1, 4 For patients who exhibit a sustained increase in heart rate despite previous adequate control, assess possible temporary or modifiable causes of worsening symptoms, such as post-operative stress or changes in alcohol consumption, prior to intensifying treatment. Also consider repeating ECG assessment and discussing these patients with a cardiologist as certain atrial tachyarrhythmias, e.g. atrial flutter, can be confused with AF and require a different approach to treatment.
Rhythm control strategies are usually initiated in secondary care
Rhythm control focuses on cardioversion using either medicines or through a controlled electric shock delivered to the heart from electrodes placed on the skin. Pharmacological cardioversion with antiarrhythmic medicines is usually trialled before considering electrical cardioversion unless the patient is haemodynamically unstable (Figure 1).1 These medicines restore sinus rhythm in around 50% of patients with recent-onset AF.4 Options include amiodarone, flecainide and sotalol.4 Balancing potential benefits against the long-term safety is a key consideration when prescribing, and medicine selection is largely dependent on patient co-morbidities.4 For example, amiodarone is preferred in patients with known LVEF dysfunction, moderate left ventricular hypertrophy or coronary artery disease,4 however, ongoing use is associated with an increased risk of thyroid, liver and pulmonary toxicity and therefore regular monitoring is required.24
 When to consider referral for further intervention
When to consider referral for further intervention
If pharmacological cardioversion strategies prove ineffective, referral for electrical cardioversion or more advanced procedures such as catheter or surgical ablation should be considered.1, 4, 5 Antiarrhythmic medicines are usually continued for at least three months following ablation to limit AF recurrence.1
Catheter ablation is being increasingly recommended in guidelines, particularly for patients with AF and HFrEF due to evidence that it improves symptoms and quality of life.3 Suitable candidates include comparatively younger patients with earlier stage HFrEF, without severe atrial myopathy and without multiple co-morbidities.3
Lifestyle modifications should be emphasised in patients with AF to help reduce symptoms and limit the risk of long-term complications, alongside their medicine regimen (Table 4).1, 25 In addition, given that many cardiovascular diseases (e.g. hypertension) and other co-morbidities increase the risk of AF recurrence and AF-related complications, detecting and managing these conditions should also be a priority.1
Table 4. Recommended goals for modifiable risk factors in patients with AF.1, 3, 4, 25
| Modifiable risk factor |
Suggested target patients with AF should try to maintain |
Hypertension |
Target blood pressure ≤ 130/80 mmHg; less assertive targets may be more appropriate in some cases, e.g. patients who are frail or with limited life expectancy |
Obesity |
Aim for a maintained weight loss of at least 10% or BMI < 27 kg/m2 |
Sedentary lifestyle |
Encourage moderate-intensity exercise, e.g. at least 150 – 210 minutes per week |
Diabetes |
Optimal glycaemic control, e.g. HbA1c < 53 mmol/mol for most patients |
Obstructive sleep apnoea |
If eligible, encourage daily use of continuous positive airway pressure |
Alcohol |
Alcohol consumption ≤ 3 standard drinks/week |
Smoking |
Smoking cessation |
N.B. Patients sometimes report caffeine as a trigger for AF.3, 25 There is no evidence that caffeine consumption is a significant cause or risk factor for AF.1, 3, 25 Lowering or limiting caffeine intake is not a required lifestyle modification for patients with AF, but could be trialled in those who report that caffeine triggers or worsens symptoms.3
Long-term involvement in endurance or high-intensity exercise increases AF risk
As a general rule, physical activity improves cardiovascular health.1 However, there is increasing evidence of an association between prolonged vigorous exercise (e.g. endurance exercise) and increased AF risk.25, 26 The incidence of AF in endurance athletes is reported as two to five times higher than non-athletes.26 While the mechanism of AF in this setting is uncertain, chronically elevated vagal tone and atrial remodelling via atrial dilatation and fibrosis are possible causes.26 The risk appears to be most significant during “middle-age” (e.g. 40 – 55 years) and a total of 1,500 – 2,000 life-time training hours of high-intensity exercise has been proposed as the risk threshold.26 Most studies have focused on male athletes.
Consider earlier opportunistic assessment for AF and a lower threshold for secondary care referral in patients who participate in endurance sports or regular high-intensity activity. In particular, those with paroxysmal AF may be considered for catheter ablation early to prevent recurrence which may better enable them to continue engaging in their sport.3 Anticoagulant medicines may not be suitable for patients who participate in contact sports, e.g. rugby or martial arts.1
Follow-up of patients with AF in primary care varies depending on the underlying pattern, co-morbidities, the medicines selected (e.g. anticoagulation, rate control, rhythm control) and how they are tolerated.3 Early follow-up is recommended initially;1, 4 a reasonable strategy could be to reassess patients after one to two weeks for medicine tolerance and whether they have reverted to sinus rhythm.1 The monitoring schedule can then be adjusted according to patient-specific factors.1, 4
At a minimum, low-risk patients with stable AF should have their stroke risk (i.e. CHA2DS2-VASc score) initially reassessed at three to six months after the index event,1 and then at least annually thereafter or sooner if there are changes in symptoms.4 However, patients with AF considered to be at higher risk or with co-morbidities will require more frequent review. For example:
- After the initial follow-up appointment some patients taking a DOAC only need monitoring every 6 – 12 months to assess renal function provided that it was normal at baseline; more frequent review may be required if baseline CrCl is reduced.3, 27 The presence of baseline liver dysfunction may also necessitate regular LFT assessment as this can influence DOAC clearance, thereby increasing the degree and/or duration of anticoagulation effect.3
- Patients taking warfarin require INR monitoring at seven days post-initiation and regular ongoing review according to their response, e.g. monthly.28 N.B. Treatment may be monitored by a pharmacist as part of the Community Pharmacy-Based Anticoagulation Management Service (CPAMS).
- Ongoing review should also be guided by baseline risk factors, e.g. patients who are frail or those starting anticoagulation with a high baseline bleeding risk might continue to require monthly appointments1
- Patients not achieving rate control targets may require more frequent assessment and dose titration in the short-term
- Cardiology guidance should support monitoring decisions for patients taking rhythm control medicines; patients taking sotalol generally require a repeat ECG the next day (due to higher risk of QT interval prolongation) and therefore it is more commonly initiated in an inpatient setting, whereas other options require reassessment between one and four weeks later1
Cardiology referral is appropriate at any point during follow-up for patients with AF who have ongoing symptoms or poorly controlled heart rate (e.g. > 110 bpm) despite appropriate escalation of pharmacological treatment.5 International guidelines recommend referral should be within four weeks of recognising the patient is not responding sufficiently to symptomatic treatments (i.e. rate or rhythm control) or following recurrence of AF after cardioversion.5
Other situations warranting cardiology referral or advice include when patients have:1, 4, 5
- Paroxysmal AF with ongoing symptoms (for consideration of ablation or other advanced treatments)
- An elevated stroke risk but long-term oral anticoagulation is contraindicated
- Symptomatic bradycardia which does not improve after reducing or withdrawing rate control medicines
- Other signs of deteriorating cardiac health, e.g. heart failure, particularly if echocardiography reveals a reduced left ventricular fraction





