In this article 
View / Download
pdf version of this article
Symptoms relating to the vulvovaginal area such as abnormal discharge, itch and pain are common, particularly for women
of reproductive age. As well as accounting for numerous general practice consultations, self-diagnosis and self-treatment
with over-the-counter products are frequent.
In women who are sexually active, history and symptoms may suggest that a physical examination and microbiological
swabs are necessary to exclude a sexually transmitted infection (STI). However, this article focuses on conditions causing
vulvovaginal symptoms in premenopausal women where, on the basis of history, STI is unlikely.
Physiological vaginal discharge
Physiological (normal) vaginal discharge is made up of a combination of mucoid secretions from the endocervical cells,
sloughed epithelial cells, vaginal transudate and products from the normal flora of the vagina, e.g. lactobacilli.1 This
discharge is characteristically white or clear and has minimal odour. It varies both in quantity and consistency between
women, during pregnancy and with the stage of the menstrual cycle. The amount of discharge, although variable, is usually
from 1 to 4 mL over 24 hours.2 During pregnancy, vaginal discharge is thicker and white-cream in colour.
For most women:
- Discharge becomes more obvious near ovulation, when it is clear, slippery and stretchy (similar to raw egg white)
for one to four days
- Discharge becomes thicker and tacky after ovulation
- Discharge is obscured once menstruation begins
- There may be little or no obvious discharge after menstruation, and then as ovulation approaches, the amount increases
again
It is important to discriminate between physiological and pathological discharge. The history of the discharge (including
onset, duration, odour, amount, presence of any intermenstrual or post-coital bleeding or discomfort), pelvic examination
and microbiological swab results will assist in making this distinction.
The acidic environment of the vagina helps prevent infection
The normal environment of the vagina and the vaginal secretions is acidic with a pH of 3.8 to 4.4.1 This
pH is maintained by the normal bacterial flora of the vagina, e.g. lactic acid-producing lactobacilli. The acidic environment
is thought to help prevent bacterial infections ascending from the lower genital tract.1 The composition of
the vaginal flora, and therefore the pH, may alter for a number of reasons such as age (e.g. the pH is more alkaline in
prepubertal children and postmenopausal women), menstruation, sexual activity (blood and semen are slightly alkaline),
contraceptive method, medicines and stress.
Alterations in the composition of the vaginal flora are usually due to overgrowth of anaerobic bacteria, which raise
the vaginal pH (more alkaline). In some women, this results in itch, swelling, discomfort and an increased vaginal discharge
that has changed in colour, consistency and odour.
Vulvovaginal hygiene
Women should be advised to avoid the use of soaps, shower gels, bubble baths, shampoos and antiseptics around the genital
area. The vulva should be gently washed with tepid or warm water. Non-soap cleansers with physiological pH (5.5) can
be used.
“Feminine hygiene” products such as washes, deodorants, powders and creams are rarely appropriate.
Vaginal douching refers to the practice of squirting water or a commercially available douching liquid up into the
vagina to wash it out. Some women use this method in an attempt to improve hygiene, particularly after menstruation or
sexual intercourse. Vaginal douching is not recommended as it alters the normal vaginal flora and may force bacteria
higher into the genital tract. It has been associated with increased risk of bacterial vaginosis, pelvic inflammatory
disease, cervicitis, endometritis, ectopic pregnancy, gonorrhoea, chlamydia, HSV and HIV infection.3,4
Bacterial vaginosis
Bacterial vaginosis (BV) results from replacement of normal vaginal flora by anaerobic bacteria such as Gardnerella,
Bacteroides and Mobilunculus species. In BV, the vaginal pH increases above 4.5 The prevalence of BV varies
widely among populations, with estimates ranging from 5 – 55 % of women.1,5 Although BV is not a STI,
it has a strong association with sexual activity and prevalence is higher in women who are sexually active (including
women who have sex with women).5,6 Other factors that may increase the incidence of BV include recent antibiotic
use, douching and use of an intrauterine contraceptive device (IUCD).5,6,7
BV is associated with an increased risk of acquiring a STI (in particular genital herpes and HIV), spontaneous miscarriage,
premature rupture of membranes, pre-term labour and infections following gynaecological surgical procedures, e.g. termination
of pregnancy or hysterectomy.5,7
Treat women with symptoms of bacterial vaginosis
BV is asymptomatic in approximately 50% of women. Treatment is not usually required in these women, except if they are
pregnant or pre-termination.8,9 Treatment is recommended for all women with symptoms of BV. However, BV spontaneously
resolves in approximately 30% of women.1,8
The most common symptom of BV is an increase in vaginal discharge, usually greyish and watery, with a characteristic
fishy odour that may be more obvious after sexual intercourse. Other symptoms such as itch, irritation or pain are uncommonly
associated with BV.8
Empiric treatment for BV may be given if:8
- There is low risk of STI (factors that increase the risk of STI include age <25 years, new sexual partner in the
last 12 months, or more than one sexual partner in the last 12 months)
- There are no other symptoms or signs that could suggest another diagnosis, e.g. itch, rash, abnormal vaginal bleeding,
fever or pain
- The woman is not pregnant or post-natal, nor recently had a miscarriage, termination of pregnancy or other gynaecological
procedure
- The symptoms are not recurrent or persistent after treatment
If the history or examination suggests empiric treatment is inappropriate, particularly if there is a risk of STI, speculum
examination and swabs (including for chlamydia and gonorrhoea) are required.8
Treatment for BV is oral metronidazole 400 mg, twice daily, for seven days or a single dose of metronidazole 2 g (5
x 400 mg). Although the single dose option is often preferred by women and may increase compliance, there is some evidence
that there is an increased risk of relapse with this dosing regimen.7,10
Adverse effects of metronidazole include nausea or other gastrointestinal disturbance, however, these effects may be
reduced if the tablets are taken with food. Women should be advised not to drink alcohol while taking metronidazole and
for a minimum of 48 hours after the course of treatment to avoid adverse effects such as flushing, headache, nausea and
vomiting.11
Ornidazole 500 mg (either single dose of 1.5 g or 500 mg, twice daily, for five days) is an effective alternative to
metronidazole for the treatment of BV.8
Treatment of male sexual partners of women with BV is not usually necessary.8,9
Treatment during pregnancy and lactation
In women who are pregnant that have symptoms of BV and the diagnosis has been confirmed with microbiology, the seven
day course of metronidazole is recommended to avoid high serum levels from the single high dose treatment.8,12 A
repeat swab should be taken after one month to check the effectiveness of the treatment.8
There are differing opinions regarding the screening and treatment of BV in asymptomatic pregnant women. Some guidelines
support treatment to reduce the risk of obstetric complications, particularly in women at increased risk (e.g. previous
miscarriage or pre-term delivery).8,9 Others argue that there is a lack of evidence for treatment and that
pregnancy outcomes are not improved after treatment.5,7 The decision to treat BV in an asymptomatic woman should
be based on her individual circumstances and discussion with her lead maternity carer.
In women who are breast feeding and have symptoms of BV, the seven day course of metronidazole is recommended to reduce
its concentration in the breast milk. The taste of breast milk may be altered by metronidazole. Some women may choose
to express and discard post-dose milk (peak serum levels occur approximately three hours after administration).11
Persistent symptoms
Treatment with metronidazole is usually effective and persistent symptoms are uncommon. If the symptoms persist after
treatment:8
- Reconsider the diagnosis (examine the patient and take appropriate swabs)
- Check compliance – ask if the whole course of metronidazole was taken
- Consider using a seven day course if the single dose regimen (2 g stat) was used
- If the patient has an IUCD, consider removal of the device and discuss other forms of contraception
Recurrent symptoms
BV is associated with high rates of recurrence. An Australian study reported recurrence rates at one month of 23% and
at one year of 58%, despite appropriate treatment.13
If the initial episode of BV was clinically characteristic (or vaginal swab results indicated BV) and metronidazole
treatment resulted in clearance of the symptoms, empiric treatment may be repeated. If the diagnosis for the initial episode
was uncertain and if an examination and swabs have not been performed, it is recommended that this is done and then treatment
can be guided by the results.
There is a lack of consistent evidence to support the use of probiotics or agents to restore vaginal acidity, including
acetic acid vaginal gel (Aci-jel), in recurrent BV.
Vulvovaginal candidiasis
Candida is present in the vaginal flora of approximately 20% of healthy women and up to 40% of women who are pregnant.7 An
overgrowth of one species, Candida albicans, is responsible for vulvovaginal candidiasis (“thrush”) in at
least 90% of affected women.7
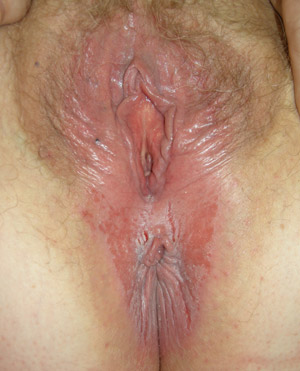 |
| Figure 1: Vulvovaginal and perianal candidiasis with erythema, oedema, fissuring and shallow erosions (Supplied by
Amanda Oakley / Dermnet NZ). |
Factors that increase the risk of vulvovaginal candidiasis include:14
- Recent use of broad spectrum antibiotics
- Pregnancy
- Diabetes
- Immunosuppression
There is no good evidence that tight or synthetic clothing or specific hygienic habits increase the risk of vulvovaginal
candidiasis.15
Vulvovaginal candidiasis is characterised by vulvovaginal itch, stinging, burning, non-specific discomfort, external
dysuria and superficial dyspareunia. If a discharge is present it is typically white, cheesy or curd-like. On examination
there may be erythema, swelling, fissuring and excoriation of the vulva (Figure 1).7,14 Signs are typically
centred on the vaginal introitus (entrance).
Treat candidiasis empirically
If the history is consistent with uncomplicated vulvovaginal candidiasis and there are no risk factors present for STI,
empiric treatment with an intravaginal antifungal cream is recommended.
Appropriate topical treatments are:
- Clotrimazole – fully funded, 2% vaginal cream for three day use or 1% for six day use
- Nystatin – fully funded, 100,000 u per 5 g vaginal cream, used twice daily for 14 days (N.B. stains underwear
yellow)
- Miconazole – partly funded, 2% vaginal cream, for seven days
Women should be advised that some vaginal creams may weaken, and therefore reduce the effectiveness of, latex condoms.
Vaginal creams can also be associated with swelling, erythema and pruritus (contact dermatitis) in some women.
Treatment with an oral antifungal (fluconazole or itraconazole) may be preferred by some women, as relief from symptoms
can be more rapid. From 1 June 2011, a single 150 mg capsule of fluconazole has been funded by endorsement, provided there
is a maximum of one capsule per prescription. The prescription must be endorsed, i.e. “certified condition” written
next to the item with a signature. N.B. Itraconazole (100 mg) and fluconazole (50 mg, 200 mg) are funded with Specialist
endorsement.
The recommended dosing regimen for oral antifungals is:12
- Fluconazole 150 mg – one capsule for one day
- Itraconazole 100 mg – two capsules, twice daily for one day OR two capsules, once daily for three days
A number of products for treating vaginal candidiasis are available OTC, including antifungal creams, pessaries and
combinations of the two. Courses range from one day to six days. A single dose OTC fluconazole pack costing approximately
$25 is also available.
Treatment of candidiasis in women who are pregnant
Women who are pregnant should be treated with intravaginal antifungals, although a longer course (up to seven days)
may be required. The woman should be advised to take care when inserting the vaginal cream using an applicator so that
there is no contact with the cervix. Some women may prefer to use vaginal pessaries (without applicator) to avoid any
risk.
Oral antifungal medicines such as fluconazole and itraconazole are best avoided during pregnancy (or in women at risk
of pregnancy).12 Both have been shown to be teratogenic in animal studies, however, pregnancy outcome data
in women and infants exposed to short courses is so far reassuring*. Fluconazole is prefered if an oral azole
antifungal must be used in a woman who is breast feeding.
* A 2019 study has found an increased risk of adverse fetal outcomes with any dose of fluconazole in pregnant women; it should be avoided.
See more here
Self diagnosis and treatment of candidiasis
It is estimated that up to 75% of women will have symptomatic Candida albicans vulvovaginitis at some stage during
their life.7 Because the symptoms are so well known, many women self-diagnose and self-treat with over-the-counter
(OTC) products. However, many of these women do not in fact have current infection - one study showed that only 33% of
women made the correct diagnosis.16 Women should be advised not to continue to use OTC products if the symptoms
do not improve with treatment or they have recurrent episodes.
When advising a woman who is seeking OTC treatment for vulvovaginal candidiasis, pharmacists may consider recommending
that the woman seeks medical advice if any of the following factors are present:14
- Age – less than 16 years or over 60 years
- First presentation of abnormal vaginal discharge
- Symptoms that are not typical, e.g. discoloured or offensive discharge, lower abdominal pain or abnormal vaginal
bleeding
- Symptoms that have not settled despite appropriate treatment
- Recurrent symptoms – more than twice in six months
- Severe or systemic symptoms
- Pregnancy
- History or concern about sexually transmitted disease
Further treatment and evaluation
There is no indication to treat the male sexual partner of a woman with uncomplicated vulvovaginal candidiasis, unless
they are symptomatic (usually short-lasting balanitis presenting with mild discomfort and erythema on the glans penis).
Physical examination and swabs (to confirm Candida and check for BV and STIs) are recommended if there are factors in
the history that suggest an alternative diagnosis, there are risk factors for STI, there is a history of recurrent episodes
(more than four in one year) or there has been any recent gynaecological intervention.17
Although treatment usually gives full resolution of symptoms within seven to 14 days, treatment failure may occur due
to:14
- Poor compliance with, or incorrect use of, medicines
- Dermatitis* due
to an exogenous irritant, e.g. soaps, shower gels or the topical antifungal cream
- Dermatitis* due
to scratching or related to irritating metabolites of the yeast
- Misdiagnosis of the initial condition
- Organisms resistant to standard treatment
- Presence of a mixed infection (such as BV, STI)
Recurrent vulvovaginal candidiasis
Recurrent vulvovaginal candidiasis, defined as four or more documented, symptomatic infections per year, occurs in 5–8%
of healthy women.14,15 The majority of cases of recurrent vulvovaginal candidiasis are due to Candida albicans,
with C. glabrata the causative strain in most other women.18 Some experts consider non-albicans candida
species to be non-pathogenic and advise against attempting to eradicate them.19 Recurrent vulvovaginal candidiasis
is thought to be due to persistent colonisation rather than episodes of new infection.20 Complete eradication of Candida is
difficult to achieve, therefore the aim of treatment for recurrent vulvovaginal candidiasis is to reduce the colonisation
of the vagina with Candida to a level where the woman is asymptomatic.20,21
In a woman with recurrent vulvovaginal candidiasis consider whether any of the following factors may be contributing:
- Risk factors for candidiasis including diabetes, frequent antibiotic use, long-term oral steroid treatment and immunosuppression14
- An alternative diagnosis, including other conditions that may cause vulval irritation, e.g. dermatitis, lichen sclerosus
or lichen planus (See below)
- The presence of a resistant species of Candida
- Oestrogen, including combined oral contraceptives or HRT. This increases vaginal glycogen, the substrate for the yeast.22 There
is no evidence that stopping the oestrogen will result in reduction in recurrent episodes,14 however, use
of progesterone-only oral contraception or intramuscular medroxyprogesterone may be useful for women with recurrent vulvovaginal
Candidia infection.23
Treatment for recurrent candidiasis
Intravaginal antifungal creams may be used for a longer course, e.g. 10–14 days,14 however, in some
women this may cause irritation or contact dermatitis. An intravaginal antifungal used before and after menstruation may
prevent recurrent symptoms.20 Oral antifungals (fluconazole or itraconazole) can be prescribed for longer courses
or taken intermittently (Table 1). N.B. Specialist endorsement is required to obtain the subsidy for these longer courses
of oral antifungal medicines.
In women with recurrent vulvovaginal candidiasis, treatment of the male partner is unlikely to be beneficial.25
There is no evidence that the ingestion or intravaginal use of Lactobacillus acidophilus (or other probiotics)
is beneficial in the treatment of vulvovaginal candidiasis.25,26 There is, however, no evidence of harm with
their use. There is no clear evidence that reducing the amount of sugar (in women without diabetes) or yeast in the diet
can help prevent recurrent episodes.
| Table 1. Induction and maintenance regimens for the treatment of recurrent vulvovaginal
candidiasis:24 |
| |
Induction |
Induction Maintenance |
| Fluconazole |
150 mg, two doses, three days apart
or
150 mg stat
or
50 mg daily for 14-28 days |
150 mg monthly for six months
or
150 mg weekly for six months |
| Itraconazole |
200 mg twice daily for one day
or
200 mg daily for three days
or
100 mg daily for 14-28 days |
100 mg weekly for six months |
| N.B: Oral antifungal medicines may rarely cause hepatotoxicity and are not indicated for use in women
who are pregnant and should be used with caution in women who are breast feeding |
Retained foreign bodies in the vagina
In adult women who present with an offensive smelling vaginal discharge, sometimes associated with intermittent spotting,
always ask about the possibility of a retained foreign body such as a tampon or condom. Some women, especially those that
use two tampons at once to absorb menstrual flow, may not realise that one of the tampons has been retained and could
be the cause of her symptoms. Normally the history of a recent period and a foul odour suggest the diagnosis, which is
then confirmed on examination.
The tampon or condom can usually be removed easily but sponge-holding forceps may be required depending on how long
the foreign body has been present and its location within the vagina. In most cases the inflammation and infection will
resolve after removal of the foreign body. There is limited evidence regarding the need for swabs and antibiotics on a
routine basis, decisions should be based on the individual clinical circumstances. If there is fever or other signs of
systemic infection, prophylactic antibiotics should be considered. A very rare complication of a retained tampon is staphylococcal
toxic shock syndrome.
Tampon related toxic shock syndrome
Staphylococcal toxic shock syndrome (TSS) was first described in 1978.27 Toxins produced by certain strains
of Staphylococcus aureus may cause potentially fatal toxic shock with symptoms such as rash, fever, hypotension,
vomiting and diarrhoea.27,28 Multi-organ system failure may rapidly develop. Although TSS may be associated
with conditions unrelated to tampon use, the environment of the vagina during menstruation favours the growth and colonisation
of tampons by staphylococci.27 Tampon related TSS is very rare, with the incidence reported to be one to three
per 100,000 women.28 Changes to the materials and methods used in tampon manufacture in the early 1980’s,
driven by a peak in the number of fatal cases, has markedly reduced the incidence of tampon-related TSS.29
Vulval itch – pruritus vulvae
Although vulval itch is a problem for many women, often embarrassment may delay seeking medical attention. Many women
are also likely to have tried to self-treat using OTC products.
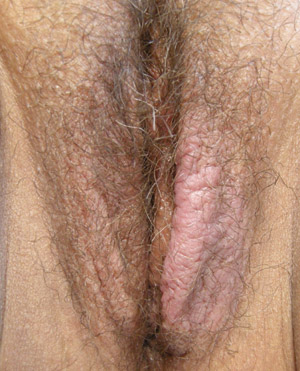 |
| Figure 2: Lichen simplex chronicus demonstrating asymmetrical uninflamed lichenification of labia
majora (Supplied by Amanda Oakley / Dermnet NZ). |
Causes of vulval itch include:
- Vulvovaginal candidiasis (See above)
- Dermatitis – most commonly contact dermatitis from exposure to irritants, e.g. soaps, perfumes, creams, barrier
contraceptives, sanitary products, urine.30 Less frequently, atopic dermatitis may occur in the vulval area.
Scratching and rubbing may lead to chronic lichen simplex (Figure 2).
- Shaving, waxing and other methods of hair removal (See below)
- Lichen sclerosus, lichen planus (See below)
- Pubic lice, thread worms, scabies (with nodules often found in the groin)
- Viral warts
- Hormonal changes which may result in atrophic vulvovaginitis, e.g. low oestrogen levels in peri and post menopausal
women and in women who are breast feeding
- Symptoms of a more generalised dermatological condition, e.g. psoriasis
- Pre-malignant or malignant condition of the vulva (rare) (See below)30
Treatment depends on identification of the underlying cause whenever possible. An emollient used both as a soap substitute
and as a moisturiser may be prescribed. Conventional oral antihistamines may help at night due to their sedative action.
Topical corticosteroids should be prescribed for women with contact dermatitis, lichen sclerosus, lichen planus and symptomatic
psoriasis. In addition, general advice may include information about avoidance of soaps, shampoos, bubble bath and other
products that may irritate or dry the skin. Occlusive underwear or tight fitting clothes may cause irritation of the vulval
area.
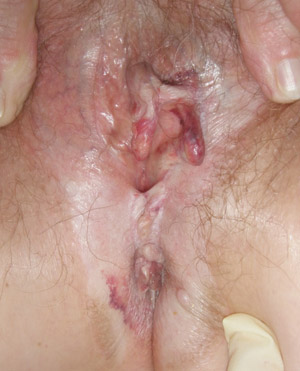 |
| Figure 3: Lichen sclerosus with typical distortion, fusion and resorption of labia minora, oedema,
ecchymosis and whitening of vulva and perianal skin (Supplied by Amanda Oakley / Dermnet
NZ). |
Lichen sclerosus
Lichen sclerosus is an inflammatory skin disorder, thought to be of autoimmune origin, which in women primarily affects
the vulval, perineal and perianal skin (but not the vagina). Although it may occur in women of any age, including prepubertal
girls, it is most frequently seen in women aged over 50 years. Symptoms include itch, which is often severe, and pain.
On examination, the skin may appear white and thickened or crinkled (Figure 3). Fissures and haemorrhages may be present.
If the diagnosis is uncertain based on the clinical appearance, biopsy may be necessary. Most cases should be referred
to a specialist in vulvovaginal disease (usually a dermatologist) for confirmation of the diagnosis, treatment and long-term
follow up.31,32
Treatment with a topical corticosteroid is not curative but aimed at reducing the symptoms to a tolerable level. Initially
a potent corticosteroid ointment (or cream if ointment is not tolerated) e.g. clobetasol (Dermol) is used, however, once
symptoms start to settle, less potent corticosteroids can be given or the frequency of application of the potent corticosteroid
can be slowly reduced. Lichen sclerosus is a chronic condition and scarring and distortion of the genital anatomy may
occur, e.g. narrowing of the vaginal entrance and resorption of the labia minora. Lichen sclerosus is also associated
with the development of vulval intraepithelial neoplasia (VIN) and invasive squamous cell carcinoma (incidence of 6%).
The vulval skin should be reviewed at least annually in women with lichen sclerosus to detect malignancy early.31,32
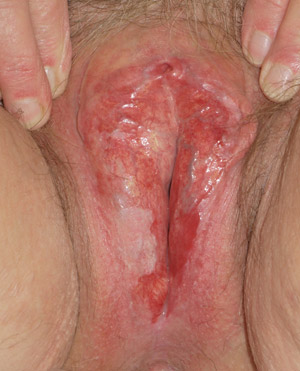 |
| Figure 4: Erosive lichen planus showing destruction and scarring of vulval skin with erosion and
atrophy of vaginal introitus (Supplied by Amanda Oakley / Dermnet
NZ). |
Lichen planus
Lichen planus is also an inflammatory skin condition of autoimmune origin with some similarities to lichen sclerosus,
however, it is less common. Unlike lichen sclerosus, lichen planus may:
- Affect other areas of the body, e.g. the oral mucosa
- Involve the vaginal mucosa
- Be only rarely seen in children
Symptoms of lichen planus are similar to those in lichen sclerosus, i.e. itch and pain. The vulval subtype of lichen
planus is often an erosive form and may cause marked pain, introital erythema and erosions (Figure 4). As with lichen
sclerosus, scarring and distortion of the affected areas may occur, but is often more severe. Women with suspected lichen
planus should be referred to a specialist in vulvovaginal disease. Diagnosis may be clear from the history and clinical
appearance, however, biopsy may be required. Treatment is initially the same as for lichen sclerosus, but lichen planus
can be very challenging to control and is more likely to require oral corticosteroids or immunosuppressive medicines.
Lichen planus is also associated with a risk of development of vulval malignancies.33,34
“Lumps and bumps”
Lump or bumps in the vulvovaginal area present in a number of ways and there is an extensive list of differential diagnoses.
Some women may present with a query about something they perceive to be abnormal which is, when examined, a variant of
normal vulvovaginal anatomy.
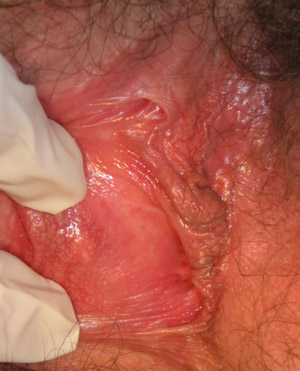 |
| Figure 5: Bartholin gland cyst arising right posterior vestibulum (Supplied by Amanda Oakley / Dermnet
NZ). |
Bartholin gland cyst or abscess
The Bartholin glands are located on each side of the vaginal opening and produce mucous to assist with lubrication of
the vagina. If the duct from the gland becomes blocked a cyst may develop within the duct (Figure 5, over page). This
produces a lump, often 1–3 cm in size, which is usually asymptomatic and does not require treatment. Cysts that
become larger may cause discomfort during sexual intercourse or when sitting or walking. A painful Bartholin abscess may
develop if the fluid within the cyst becomes infected.
Depending on the size and severity of symptoms, treatment options include warm compresses, saline baths, incision and
drainage under local anaesthetic or excision or marsupialisation of the gland under sedation or general anaesthetic.35 Although
incision and drainage is the most frequently performed procedure, it is associated with a high rate of recurrence.35 Oral
antibiotics are not indicated unless there is associated cellulitis or systemic symptoms.35 If required, broad
spectrum antibiotic cover is necessary as the infection is usually polymicrobial.
Carcinoma of the Bartholin gland is rare (approximately 1% of genital malignancies in women), however, this diagnosis
should be considered in a woman aged over 40 years. Features consistent with carcinoma of Bartholin gland include a mass
that is:35
- Painless
- Fixed to the underlying tissues
- Solid – however the mass may also be cystic, abscessed or only partially solid
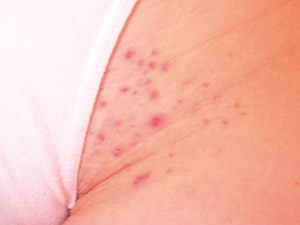 |
| Figure 6: Folliculitis due to shaving pubic hair (Supplied by Amanda Oakley / Dermnet
NZ). |
Complications of pubic hair removal
There are many methods used for hair removal such as shaving, depilatory creams, electrical epilation, waxing, electrolysis,
light and laser devices. Complications may include infection, ingrown hairs and contact dermatitis. Most hair removal
methods cause microtrauma to the skin and allow introduction of bacteria from the skin or from items used in hair removal.36 Shaving
or waxing the pubic hair may cause irritation of the skin or folliculitis (bacterial infection of the hair follicles within
the epidermis which presents as multiple papules and pustules that may be itchy or uncomfortable (Figure 6). An abscess
may also develop and usually presents as an isolated tender lump, sometimes with surrounding cellulitis.
Folliculitis may resolve spontaneously with conservative treatment (warm compresses, saline baths) although topical
antibiotics may be required in some cases. If there is fever, other systemic symptoms, or the woman is at increased risk
because of co-morbidities such as diabetes or immunosuppression, oral antibiotics (e.g. flucoloxacillin) should be prescribed.36 Treatment
of an abscess will normally require incision and drainage and/or oral antibiotics depending on the clinical presentation.
Hair removal, pressure or irritation from tight clothing and the tendency for the pubic hair to be coarser and curlier,
increase the likelihood of ingrown hairs. A warm compress can be held over the affected area and then the hair lifted
free of the skin with a sterile needle. If infection develops, topical antibiotics may be required.
Benign vs malignant vulval skin lesions
Lesions of the vulval area may be benign, pre-malignant or malignant. Malignant lesions may not cause symptoms and there
also may not be an obvious mass. Women, who present with chronic vulval itch or irritation, particularly if there is no
apparent reason, should be referred for colposcopy and biopsy. Symptoms of itch, a burning sensation or pain are associated
with malignant vulval lesions in approximately 50% of women.37,38
The clinical features used to help distinguish benign from malignant skin lesions anywhere on the body, are also those
that may raise suspicion of a vulval malignant skin lesion. These features include:
- Asymmetry
- Irregularity of the border
- Change in colour
- Increase in size
- Bleeding or lack of healing
- Failure to respond to appropriate treatment
Benign skin lesions
Numerous types of benign skin lesions may be found in the vulvovaginal area including:
- Lipomas
- Seborrhoeic keratoses
- Melanocytic naevi
- Skin tags
- Fordyce spots (ectopic sebaceous glands)
- Molluscum contagiosum
- Various rare adnexal neoplasms
Malignant skin lesions
Although most malignancies involving the vulval area occur in postmenopausal women, vulvar intraepithelial neoplasia
(VIN) may begin in women aged 30 to 40 years.39 The lesions may be asymptomatic found during an examination
for a routine cervical smear or other unrelated reason. VIN has the potential to progress to invasive carcinoma of the
vulva and women with suspicious lesions require referral to secondary care for biopsy and treatment.
Women with symptomatic vulval invasive cancers may present with itch, an obvious lump, pain, ulceration or bleeding.
The inner edges of the labia are the most common site for vulval cancer.38 Approximately 90% of vulval cancers
are squamous cell carcinomas, however, other types of malignant lesion may occur in the vulval area such as melanoma,
basal cell carcinoma, sarcoma and rarely, adenocarcinoma of the Bartholin gland.37
Risk factors for vulval cancer include smoking, VIN, lichen sclerosus, lichen planus, previous HPV infection and positive
HIV status.37,39
Vulval Pain
Chronic vulval pain is estimated to affect 16% of women at some point in their life.40 This is thought to
be a conservative estimate of the lifetime prevalence because many women do not seek medical assistance. Women of all
ages may be affected, however, it is more common in women of reproductive age. It is frequently associated with sexual
dysfunction.
Pain in the vulval area is classified according to whether it is:40
- Due to a specific disorder – pain from infection, inflammation, neoplasia and neurologic causes
- Without any evidence of a specific disorder – burning discomfort of the vulva is described as vulvodynia. This
may be localised to the introitus (vestibulodynia) and triggered by contact, e.g. intercourse, or generalised and persistent
when it is more likely to have a neuropathic origin.
A comprehensive history and physical examination is needed to make an accurate diagnosis of the cause of the pain. A
careful assessment of urological, pelvic and anorectal systems should be included. Treating any underlying disorder usually
resolves the pain. Vulvodynia may be difficult to manage and consultation with a specialist is recommended. Strategies
include an assessment of the pelvic floor, rarely the use of topical medicines (e.g. topical local anaesthetic gels at
night and also prior to sexual intercourse) and oral medicines such as tricyclic antidepressants and gabapentin.40
Vulvovaginal conditions in pre-pubertal girls
Young girls may present with vulvovaginal symptoms such as itch, discomfort, erythema, vaginal discharge or bleeding.
Contributing factors include:41
- Low oestrogen levels, resulting in thinner vaginal epithelium
- Less acidic vaginal pH
- Flattened and thinner labia providing a reduced physical barrier to infection
- Close proximity to the anus, particularly once the child is becoming more independent with toileting and washing
A comprehensive history should be obtained and should include questions about the symptoms (type, timing, site), toileting
(both bowel and bladder) and perineal hygiene, recent medicines, atopy and, if abuse is suspected, enquiry about social
circumstances, i.e. who the caregivers are and what the daily routine of the child is, e.g. daycare, school.42
An external physical examination and in some circumstances a swab of vaginal discharge if present, taken from the introitus,
may assist in making the diagnosis although in many cases, no clear cause is identified and this is termed non-specific
vulvovaginitis.
The majority of vulvovaginal conditions in prepubertal girls are secondary to local irritants causing contact dermatitis,
inflammation or infection, often related to hygiene as independence is reached. In younger girls, insertion of foreign
bodies into the vagina (e.g. toilet paper, beads or marbles) may cause an offensive, blood stained discharge.
Examples of other conditions that may cause vulvovaginal irritation include:
- Candida infection which occurs in 3–4 % of prepubertal girls.43 Although Candida is an
important contributor to napkin dermatitis in infants, in older children, infection should be confirmed prior to treatment.
Over-diagnosis is common.
- Thread worms, which should be considered if there is nocturnal itch especially if the perianal area is also involved
- Sexual abuse, which may result in a sexually transmitted infection or trauma to the vulvovaginal area
- Chlamydia or Human papillomavirus (HPV) from maternal-child transmission at birth in infants and young children
- Lichen sclerosus, which may present with itch, discharge, discomfort, bowel or bladder symptoms and bleeding and
responds to topical corticosteroid (specialist assessment is required)
- Trauma from accidents during sport or playground activities (“straddle” injuries) which may cause significant
bleeding. Check that the physical injuries correlate with the history of the accident, i.e. affect the anterior structures
(vulva, mons, clitoral hood) rather than the more posterior structures (posterior fourchette and hymenal area)43
Acknowledgement
Thank you to Dr Amanda Oakley, Specialist Dermatologist, Clinical Associate Professor, President of
the Australian and New Zealand Vulvovaginal Society, Tristram Clinic, Hamilton and Professor Cindy Farquhar,
Department of Obstetrics & Gynaecology, Faculty of Medicine and Health Sciences, University of Auckland for expert
guidance in developing this article.
References
- Mylonas I, Bergauer F. Diagnosis of vaginal discharge by wet mount microscopy: A simple and underrated method. Obstet
Gynecol Surv 2011; 66(6):359-68.
- Sobel J. Diagnostic approach to women with vaginal discharge or vulvovaginal symptoms. UpToDate 2011. Available from: www.uptodate.com (Accessed
Oct, 2011).
- Klebanoff M, Nansel T, Brotman R, et al. Personal hygienic behaviours and bacterial vaginosis. Sex Transm Dis 2010;37(2):94-9.
- Tsai C, Shepherd B, Vermund S. Does douching increase risk for sexually transmitted infections? A prospective study
in high-risk adolescents. Am J Obstet Gynecol 2009;200:38.e1-e8.
- Donders G. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a
review. Obstet Gynecol Surv 2010;65(7):462-73.
- Verstraelen H, Verhelst R, Vaneehcoutte M, Temmerman M. The epidemiology of bacterial vaginosis in relation to sexual
behaviour. BMC Infect Dis 2010;10:81.
- Sherrard J, Doners G, White D, Jensen J. European (IUSTI/WHO) guideline on the management of vaginal discharge, 2011.
Int J STD AIDS 2011;22:421-9.
- Clinical knowledge summaries (CKS). Bacterial vaginosis. CKS, 2009. Available from: www.cks.nhs.uk (Accessed
Oct, 2011).
- New Zealand Sexual Health Society (NZSHS). Bacterial vaginosis. NZSHS, 2009. Available from: www.nzshs.org (Accessed
Oct, 2011).
- Koumans E, Markowitz L, Hogan V and CDC BV Working Group. Indications for therapy and treatment recommendations for
bacterial vaginosis in nonpregnant and pregnant women: a synthesis of data. Clin Infect Dis 2002;35(suppl 2):s152-72.
- Mylan New Zealand Ltd. Trichozole: metronidazole. Medicine data sheet. Available from: www.medsafe.govt.nz (Accessed
Nov, 2011).
- British National Formulary (BNF). BNF 62. London: BMJ Publishing Group and Royal Pharmaceutical Society of Great
Britain, 2011.
- Bradshaw C, Morton A, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months
after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006;193:1478-86.
- Clinical Knowledge Summaries (CKS). Candida – female genital. CKS, 2010. Available from: www.cks.nhs.uk (Accessed
Oct, 2011).
- Sobel JD. Vulvovaginal candidosis. Lancet 2007;369:1961-71.
- Ferris D, Nyirjesy P, Sobel J, et al. Over-the-counter antifungal drug misuse associate with patient-diagnosed vulvovaginal
candidiasis. Obstet Gynecol 2002;99:419-25.
- Clinical Knowledge Summaries (CKS). Vaginal discharge. CKS, 2009. Available from: www.cks.nhs.uk (Accessed
Oct, 2011).
- Sobel J, Wiesenfeld H, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis.
N Engl J Med 2004;351:876-83.
- Dennerstein G, Ellis D, Reed C, Bennett C. Pathogenicity of non-albicans yeasts in the vagina. J Low Genit Tract
Dis 2011;15(1):33-6.
- Dermnet NZ. Vulvovaginal candidiasis. Available from: www.dermnetnz.org (Accessed
Oct, 2011).
- Watson C, Calabretto H. Comprehensive review of conventional and non-conventional methods of management of recurrent
vulvovaginal candidiasis. Aust NZ J Obstet Gynaecol 2007;47;262-72.
- Dennerstein G, Ellis D. Oestrogen, glycogen and vaginal candidiasis. Aust NZ J Obstet Gynaecol 2001;41(3):326-8.
- Fiden P, Cutright J, Steel C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun
2000;68(2):651-7.
- New Zealand Sexual Health Society (NZSHS). Recurrent vulvovaginal candidiasis. NZSHS, 2009. Available from: www.nzshs.org (Accessed
Oct, 2011).
- Spence D. Candidiasis (vulvovaginal). Clin Evid 2010. Available from: www.clinicalevidence.bmj.com (Accessed
Oct, 2011).
- Falagas M, Betsi G, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J. Antimicrob
Chemother 2006;58(2):266-72.
- Tang Y, Himmelfarb E, Wills M, Stratton C. Characterisation of three Staphylococcus aureus isolates from a 17-year-old
female who died of tampon-related toxic shock syndrome. J Clin Microbiol 2010;48(5):1974-7.
- Schlievert P, Nemeth K, Davis C, et al. Staphylococcus aureus extotoxins are present in vivo in tampons. Clin Vacc
Immunol 2010;17(5):722-7.
- Meadows M. Tampon Safety. TSS not rare, but women still should take care. FDA Consumer magazine. March-April 2000.
Available from: www.fda.gov (Accessed Oct, 2011).
- Clinical knowledge summaries (CKS). Puritus vulvae. CKS, 2011 Available from: www.cks.nhs.uk (Accessed
Oct, 2011).
- Dermnet NZ. Lichen sclerosus. Available from: www.dermnetnz.org (Accessed
Oct, 2011).
- Dalziel K, Shaw S. Easily missed? Lichen sclerosus. BMJ 2010;15;340:c731.
- Dermnet NZ. Lichen planus. Available from: www.dermnetnz.org (Accessed
Oct, 2011).
- McPherson T, Cooper S. Vulval lichen sclerosus and lichen planus. Dermatol Ther 2010; 23:523–32.
- Pundir J, Auld B. A review of the management of diseases of the Bartholin’s gland. L Obstet Gynaecol 2008;28(2):161-5.
- Dendle C, Mulvey S, Pyrlis F, et al. Severe complications of a “Brazilian” bikini wax. Clin Inf Dis.
2007;45:e29-31.
- Elkas J, Berek J. Vulvar cancer: clinical manifestations, diagnosis, and pathology. UpToDate, 2011. Available from: www.uptodate.com (Accessed
Oct, 2011).
- Dermnet NZ. Vulval cancer. Available from: www.dermnetnz.org (Accessed
Oct, 2011).
- Lai K, Mercurio M. Medical and surgical approaches to vulvar intraepithelial neoplasia. Dermatol Ther 2010;23:477-84.
- Danby C, Margesson L. Approach to the diagnosis and treatment of vulvar pain. Dermatol Ther 2010;23:485-504.
- Garden AS. Vulvovaginitis and other common childhood gynaecological conditions. Arch Dis Child Educ Pract Ed 2011;96:73-8.
- Dei M, Di Maggio F, Di Paolo G. Vulvovaginitis in childhood. Best Pract Res Clin Obstet Gynaecol 2010;24:129-37.
- Laufer M, Emans S. Vulvovaginal complaints in the prepubertal child. UpToDate, 2009. Available from: www.uptodate.com (Accessed
Oct, 2011).