Dabigatran has been available for general practitioners to prescribe since July, 2011. Dabigatran is indicated for prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation, and for venous thromboembolism prophylaxis after major orthopaedic surgery (specifically hip and knee replacement). There is currently no evidence that it should be used for indications other than these. Non-haemorrhagic gastrointestinal adverse effects (primarily dyspepsia) are the most frequently reported adverse reaction to dabigatran, although bleeding, as with any anticoagulant medicine, remains one of the main risks. There have been no reports of new adverse effects emerging since dabigatran has been used in general practice.
In this article 
View
/ Download pdf version of this article
Dabigatran has been available for general practitioners to prescribe since July, 2011. Twelve months later, over 14
000 patients were being dispensed this medicine. Dabigatran is indicated for prevention of stroke and systemic embolism
in people with non-valvular atrial fibrillation, and for venous thromboembolism prophylaxis after major orthopaedic surgery
(specifically hip and knee replacement). There is currently no evidence that it should be used for indications other
than these. Non-haemorrhagic gastrointestinal adverse effects (primarily dyspepsia) are the most frequently reported
adverse reaction to dabigatran, although bleeding, as with any anticoagulant medicine, remains one of the main risks.
There have been no reports of new adverse effects emerging since dabigatran has been used in general practice.
Dabigatran now commonly used in general practice
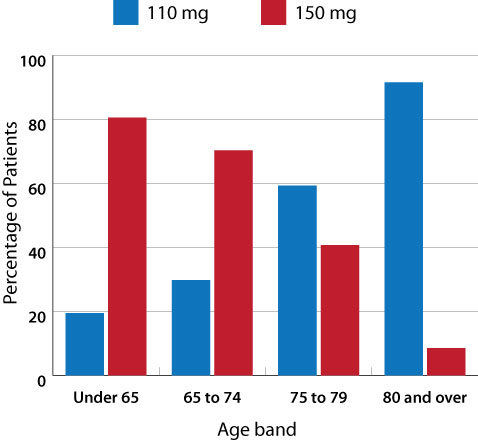
Figure 1: Percentage of patients dispensed dabigatran by age and dose, from July 2011 to June 2012
(Pharmaceutical Warehouse data)1
The oral anticoagulant, dabigatran etexilate, has been available, fully subsidised, on the Pharmaceutical Schedule since
July, 2011. Between July 2011 and June 2012, dabigatran was dispensed over 95 000 times to more than 14 000 patients in
New Zealand. Usage is continuing to increase with between 8000 and 9000 new dispensings each month.1
Dabigatran is available in two formulations – 110 mg and 150 mg. The lower dose (2 x 110 mg/day) is recommended in people
aged over 80 years and those with renal impairment (creatinine clearance 30 – 50 mL/min). Figure 1 shows
that most people aged over 80 years are being dispensed the 110 mg formulation of dabigatran, however, just under 10%
of people in this age group are receiving a dose that is higher than recommended.1
Indications for dabigatran are unchanged
Dabigatran is indicated for prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation,
and for venous thromboembolism (VTE) prophylaxis after major orthopaedic surgery (specifically hip and knee replacement).
The specific indication for dabigatran for the prevention of stroke in patients with non-valvular atrial fibrillation
requires at least one other risk factor for stroke, e.g. previous transient ischaemic attack or stroke, left ventricular
ejection fraction < 40%, symptomatic heart failure, age ≥75 years, age ≥65 years plus diabetes or hypertension or coronary
artery disease.9
Dabigatran is NOT indicated for use in people with valvular heart disease or mechanical heart valves.
Dabigatran has not been evaluated in people with bioprosthetic valves and therefore should also NOT be
used in this situation.2
Thrombosis of mechanical heart valves in patients taking dabigatran
Despite not being indicated for use in patients with mechanical heart valves, New Zealand data from the Centre for Adverse
Reactions Monitoring (CARM) show six reports of patients with mechanical values experiencing adverse effects due to the
use of dabigatran. Published reports associating dabigatran with valvular thrombosis, leading to valvular dysfunction,
in patients with mechanical valves are appearing in the literature.3–5 In the New Zealand case reports, both
patients had mechanical aortic valves and had previously been anticoagulated with warfarin. The patients were compliant
with dabigatran treatment, however, they developed thrombosis on the prosthetic valve. One patient also developed multiple
embolic cerebral infarctions.4
Although evidence from in vitro studies has suggested that dabigatran may prevent thrombosis on mechanical valves, dabigatran
has never been indicated for use in this clinical setting.5, 6 There is speculation that when dabigatran is
used in patients with mechanical valves, at doses currently recommended for non-valvular AF, it does not prevent formation
of thrombus. A clinical trial that was underway to assess the suitability of dabigatran for people with mechanical heart
valves has been halted (December, 2012) due to higher than expected numbers of thromboembolic events (including stroke,
valve thrombosis and myocardial infarction) occurring in study participants taking dabigatran (see “RE-ALIGN trial – now
halted”).7, 8
The RE-ALIGN trial – now halted
The RE-ALIGN trial (Randomised phase II to Evaluate the sAfety and
pharmacokinetics of oraL dabIGatran etexilate in patients after heart valve replacemeNt)
began in late 2011 to investigate whether dabigatran could be used as an alternative to warfarin for patients with mechanical
heart valves. Participants were randomised to warfarin or dabigatran (150, 200 or 300 mg, twice daily, based on their
creatinine clearance) either at the time of their surgery or three months later.
In October, 2012, the immediate post-surgical arm of the trial was stopped. In December, 2012, the manufacturer issued
a press release announcing that they had made the voluntary decision to halt the entire trial because the “investigated
dosing regimen did not achieve the desired results”.8 Results provided to the FDA by the manufacturer reported
higher than expected numbers of thromboembolic events in participants taking dabigatran compared to participants taking
warfarin.2 It has been suggested that this may have been due to the higher thrombotic risk that accompanies
the early post-surgical period, however, events also occurred in patients who had valve replacement surgery more than
three months previously.2, 7 Preliminary results from the manufacturer also report that patients taking dabigatran
had significantly more events of major bleeding (22.5%) than patients taking warfarin (13.5%).2
As a direct result of the problems occurring during this trial, both the Food and Drug Administration (FDA) in the
USA and the European Medicines Agency (EMA) have now stated that dabigatran is contraindicated in people with mechanical
heart valves.2
Dabigatran dosing recommendations have not changed
For the prevention of stroke in people with non-valvular atrial fibrillation the recommended dose of dabigatran is:9,
10
- 150 mg, twice daily, provided creatinine clearance >30 mL/min
- 110 mg, twice daily, for patients aged ≥80 years
In addition, in patients aged 75 – 80 years and patients with creatinine clearance 30 – 50 mL/min consider using 110
mg, twice daily, if their risk of bleeding is high and their risk of thrombosis is low.
Stroke and bleeding risk in patients with AF should be assessed using stroke assessment tools, e.g. CHADS2, CHA2DS2-VASc
and HAS-BLED.
For further information, including the stroke assessment tools,
 see “The use of antithrombotic
medicines in general practice”, BPJ 39 (Oct, 2011).
see “The use of antithrombotic
medicines in general practice”, BPJ 39 (Oct, 2011).
Why calculate creatinine clearance?
Dabigatran is predominantly renally excreted therefore any deterioration in renal function will increase the concentration
of dabigatran and increase the risk of adverse effects, primarily bleeding. The Modification of Diet in Renal Disease
(MDRD) eGFR calculation reported by laboratories in New Zealand may be inaccurate in older people or people with a BMI < 18.5kg/m2
or > 30 kg/m2. When a patient’s creatinine level rises above the normal range, alterations to medicine doses may be required.It
is recommended that glomerular filtration rate is estimated using the Cockcroft-Gault equation rather than relying on
the laboratory supplied MDRD eGFR. Tools for calculating creatinine clearance using the Cockcroft-Gault equation are available
online online (e.g.
http://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation/ )
or can be downloaded for use on portable devices such as smart phones or tablet devices.
Dabigatran for VTE prophylaxis after major joint surgery
For the prophylaxis of VTE following major orthopaedic surgery the recommended dose of dabigatran is:9
- 220 mg (2 × 110 mg tablets), once daily, for patients with creatinine clearance > 50 mL/min
- 150 mg ( 2 × 75 mg tablets), once daily, for patients with creatinine clearance 30 – 50 mL/min
N.B. The length of the course varies with the type of surgery – knee replacement surgery ten days, hip replacement surgery
35 days.9
Evidence suggests that treatment with oral dabigatran, compared with subcutaneous low molecular weight heparin (LMWH),
for the prophylaxis of VTE after knee or hip replacement surgery is well tolerated and associated with high levels of
patient and clinician satisfaction.11–13 The efficacy and safety of dabigatran is comparable to other anticoagulants
used for VTE prophylaxis.12,14 The key advantage of dabigatran in this setting is the use of a fixed dose given
by the oral route. One practical advantage of the oral route of administration is that patients do not have to self-administer
subcutaneous doses of LMWH after discharge from hospital. Disadvantages include nausea, an inability to tolerate oral
medicine in the early postoperative period, increased post-operative wound discharge and limitations in use in patients
with spinal anaesthesia.12
Should you prescribe warfarin or dabigatran?
Although dabigatran is likely to be more convenient and simpler to use than warfarin, it is not suitable for all people
and all situations.
The advantages of dabigatran compared with warfarin include:
- More convenience as frequent INR tests and dose adjustments are not needed
- Effective anticoagulation in patients who have previously been difficult to control on warfarin (as long as poor adherence
is not the reason for the unstable INR levels)
- Fewer drug and dietary interactions than warfarin
- Reduction in the risk of intracranial haemorrhage15
Some people, however, who were changed from warfarin to dabigatran, have now changed back. Reasons suggested for this
include:
- “Missing” the reassurance of knowing that they have effective anticoagulation, i.e. their INR is within the therapeutic
range (this applies to both patient and clinician)
- Patients finding the twice daily dosing difficult (and therefore risking ineffective anticoagulation because of the
short half-life of the medicine, approximately 12–14 hours in a patient with normal renal function)
- Presence of adverse effects from dabigatran, particularly dyspepsia (there is anecdotal evidence that this may affect
up to 30% of patients)
- Deteriorating renal function where eGFR drops close to 30 mL/min
Changing from warfarin to dabigatran requires a different “mindset” with regards to effective dosing and anticoagulation.
Both clinicians and patients need to be comfortable with the concept that while taking dabigatran they do not need blood
tests to check its effectiveness as an anticoagulant. Ensuring that patients are well informed about dabigatran when it
is initiated is likely to assist with adherence. Reminders can be put in place, e.g. a mobile phone alert, to help patients
remember the twice daily doses. If a dose is missed, another capsule should be taken as soon as the patient remembers,
provided there is more than six hours before the next dose.
How to initiate dabigatran
Prior to initiating dabigatran, all patients should have an assessment of renal function.9 Dabigatran is
contraindicated in people with a calculated creatinine clearance of < 30 mL/min. People with moderate renal impairment
(30 – 50 mL/min) are at increased risk of bleeding and dabigatran should be used with caution.
In a patient not previously anticoagulated with warfarin, dabigatran is started at the appropriate dose depending on
age and renal function and is continued at the same dose. There is no need for a loading dose to be given.
In a patient already anticoagulated with warfarin, the warfarin should be stopped and the INR monitored. Dabigatran
can be started at the appropriate dose for age and renal function when the INR is < 2.0.
An iPhone application is available for use when initiating dabigatran
An application for smart phones has been developed by Dr Paul Harper, Clinical Haematologist, Medlab Central. It is
designed to help determine the dose of dabigatran that should be used in patients with non-valvular AF or for orthopaedic
prophylaxis. The application gives a recommended dose for each indication based on the patient’s age and renal function.
It also includes relevant drug information (tablet sizes, pharmacology, storage and advice about taking the medicine),
specific dosing instructions and information about adverse effects, interactions and actions to take if a patient is
bleeding.
 The application “Managing Dabigatran – guidelines for the management of dabigatran”
is available for free: Search App Store, keyword: dabigatran.
The application “Managing Dabigatran – guidelines for the management of dabigatran”
is available for free: Search App Store, keyword: dabigatran.
How to change a patient from dabigatran back to warfarin
Check the patient's creatinine clearance, unless this has been done within the last few weeks. If the creatinine clearance
is > 50 mL/min, warfarin treatment should be started three days before discontinuing dabigatran at a dose similar to the
patient’s previous dose. If the creatinine clearance is 30 – 50 mL/min, initiate warfarin two days before stopping dabigatran.9 INR
should be checked regularly until stable, when the frequency between tests can be extended.
How to temporarily stop dabigatran for a planned surgical procedure
Patients who have a creatinine clearance > 50 mL/min, should discontinue dabigatran 24 hours before the planned surgical
procedure. In patients with a high risk of bleeding or if a major surgical procedure is planned, dabigatran should be
discontinued two days before the procedure. Patients with a creatinine clearance of 30 – 50 mL/min should discontinue
dabigatran two to four days before the planned procedure, because the clearance of dabigatran is likely to be prolonged.9
Renal function must be monitored in people taking dabigatran
In addition to assessing baseline renal function, regular monitoring of renal function is important in the majority
of patients taking dabigatran. Renal function should be assessed:9
- For all people prior to the initiation of dabigatran
- For people taking dabigatran who have a change in their clinical situation that may be associated with a decline in
renal function e.g. dehydration, diuretic usage or hypovolaemia
- At least annually in all people taking dabigatran who are aged over 75 years
- At least annually (but preferably three to six monthly) in all people taking dabigatran who have a creatinine clearance
of 30 – 50 mL/min
Laboratory tests are not needed to guide dosing decisions
Unlike warfarin, dabigatran has a wide therapeutic window, predictable pharmacokinetics and pharmacodynamics and a rapid
onset of action therefore doses are standardised and monitoring for effectiveness is not required. In addition, there
is no readily available, effective laboratory test that can be used to guide the dosage of dabigatran or to assess the
effectiveness of the medicine. If bleeding occurs in a patient taking dabigatran, the medicine should be stopped and in
some situations (usually in secondary care) laboratory investigation using a combination of tests such as thrombin time,
activated partial thromboplastin time, fibrinogen and ecarin clotting time (if available) may assist with management.
Is dabigatran RELY-ABLE?
Evidence for the effectiveness and safety of dabigatran as an oral anticoagulant for use in patients with non-valvular
AF was based predominantly on the Randomised Evaluation of Long-Term Anticoagulation therapy (RE-LY) trial.16,17 Multiple
studies have been published since, but of key interest is the long-term extension study which has followed patients who
participated in the RE-LY study, who have continued with dabigatran treatment. The RELY-ABLE trial was designed to establish
the long-term safety of dabigatran in patients with non-valvular AF and also to assess efficacy outcomes.
Preliminarily results from the 5851 patients followed in the RELY-ABLE trial appear to support the findings of the
RE-LY trial with a net clinical benefit from both the 110 mg and 150 mg dose.18 The 150 mg dose continues
to be associated with a higher rate of major bleeding than the 110 mg dose.17
However, important limitations of this study are that only 32% of patients from RE-LY were included, participation
was voluntary and follow-up was stopped when the medicine was stopped. Only 12% of patients from RE-LY were followed
for a further 28 months, and they were likely to be slightly younger, more likely to have permanent AF and less likely
to have heart failure.17 Whether these differences between study participants and the other limitations of
the RELY-ABLE trial will have any clinical significance in the longer term is not known. Comparisons with warfarin are
also not available as patients who had been randomised to warfarin in RE-LY were not included in RELY-ABLE.
No new adverse effects identified to date
In the three months after July, 2011, when dabigatran was included on the Pharmaceutical Schedule, there were multiple
reports submitted to CARM (approximately 70–100 per month); however, since December 2011, the number of reports has dropped
dramatically.19 This may in part be explained by a tendency for clinicians not to report on a drug that is
no longer “new”. An analysis of reports to the end of February 2012 is shown in Table 1.
Table 1: Overview of types of suspected adverse reactions reported19
| Grouping |
Number of Reports (n=345) |
% of Total Reports |
| Bleeding |
139 |
40.3 |
| GI Symptoms (non-haemorrhagic) |
145 |
42.9 |
| Thromboembolic |
14 |
4.1 |
| Events secondary to inappropriate use |
*28 |
8.5 |
* includes six reports where dabigatran was used in a patient with a mechanical valve
Non-haemorrhagic gastrointestinal adverse effects (primarily dyspepsia) are the most frequently reported adverse drug
reaction, although bleeding, as with any anticoagulant medicine, remains one of the main adverse risks of dabigatran.
There were more adverse effects in older people, but this also reflects increased use of dabigatran in older people. This
pattern, however, does not apply to patients aged over 80 years, where the number of reports was higher than would be
expected for the usage in this age group.19 Since the last published Medsafe update in February, 2012, CARM
reports that there have not been any newly emerging adverse effects associated with dabigatran.
Minimising adverse effects
The key adverse effects in the CARM reports include bleeding, non-haemorrhagic gastrointestinal effects, thromboembolic
events and events secondary to inappropriate use. In general, when initiating dabigatran always consider; the age of the
patient, that the indication is appropriate, that the patient’s renal function (creatinine clearance) has been checked
and that all the patient’s medicines have been reviewed.
The risk of bleeding can be minimised by ensuring that:
- The dose used is not higher than recommended for the patient’s age (particularly in people in the >80 years age group)
or renal function
- In patients already anticoagulated with warfarin their INR is < 2.0 prior to the initiation of dabigatran
- Dabigatran is used with caution with medicines that may increase bleeding risk, e.g. aspirin, clopidogrel, dipyridamole
and NSAIDs. Ensure also that the patient does not continue taking warfarin.
Other new oral anticoagulants
Dabigatran (an oral direct thrombin inhibitor) is only one of a number of new oral anticoagulant medicines becoming
available worldwide. Rivaroxaban and apixaban (both oral, direct factor Xa inhibitors) are being increasingly used internationally
for prevention of both stroke and VTE. There is evidence from clinical trials that these medicines may have some advantages
and be associated with lower morbidity and mortality than dabigatran, however, further research, in particular head-to-head
trials, is required.22–24 At present, although rivaroxaban is available in New Zealand, it is only subsidised
under Special Authority for the prophylaxis of VTE in patients after hip and knee replacement surgery and apixaban is
not yet available in New Zealand.
The risk of non-haemorrhagic gastrointestinal effects, primarily dyspepsia, can be minimised by advising
patients to take dabigatran with food. If required, a proton pump inhibitor (PPI)* or H2 antagonist can be prescribed,
however, patients should be advised that if the dyspepsia persists, they should return for review.
*Although there is evidence from clinical trials that co-administration of a PPI reduces the plasma concentration of
dabigatran, it appears that this interaction is not clinically significant.20
The risk of thromboembolic events can be minimised by ensuring adherence to the twice daily dosing
that is required for effective anticoagulation.
The risk of events secondary to inappropriate use can be minimised by:
- Not using dabigatran in patients with a creatinine clearance of < 30 mL/min
- Not using dabigatran in patients with mechanical heart valves (including bioprosthetic valves)
- Using lower dose dabigatran (110 mg, twice daily) in older patients and those with moderate renal impairment (30 –
50 mL/min)
- Considering age, renal impairment and bleeding risk when determining the correct dose of dabigatran
Dabigatran has also been associated with a possible increased risk of myocardial infarction (MI).16 Although
researchers continue to debate whether this reflects a true risk of dabigatran use or a protective effect from warfarin,
a recent meta-analysis concluded that dabigatran was associated with an estimated 27 – 33% increase in relative risk of
MI or acute coronary syndrome, although the absolute risk was small (0.27%).21
This information was updated December 2014
Dabigatran now in blister strips
Dabigatran (Pradaxa) capsules were originally packaged loosely in bottles and due to the hydroscopic properties of the medicine,
the manufacturer recommended that the capsules be stored in their original bottle. Dabigatran is now available in blister strips, which
means that dabigatran can be put into pharmacy generated blister packaging, however, several practical issues remain:
- The capsules must be kept in the original manufacturers foil and when the foil is cut around the capsule to allow it to
fit it into the blister, the seal around the capsule must remain intact. Because of the size of the capsule and cut foil, a
tray with a large blister will be needed. Folding or bending the foil to make it fit can compromise the seal around the capsule
- There may not be space for additional tablets/capsules in the blister due to the size of the capsule and the cut foil,
which may mean extra packs for patients and therefore potentially extra cost
- Packs with 24mm blisters are widely available, however, newer products have recently been developed with
a significantly increased blister volume
It is recommended, therefore, that packaging options for dabigatran are discussed with a pharmacist
so that a practical solution can be found that suits the patient’s individual needs.
ACKNOWLEDGEMENT: Thank you to members of the anti-thrombotic consensus group
(Professor Carl Burgess, Dr John Fink, Dr Sisira Jayathissa, Associate Professor Stewart Mann, Mr Allan Panting,
Dr Jim Vause and Dr Howard Wilson) for expert guidance in developing this article.
References
- Ministry of Health. Pharmaceutical collection. Wellington: Ministry of Health; 2012.
- Food and Drug Administration. FDA drug safety communication: Pradaxa (dabigatran etexilate mesylate) should not be
used in patients with mechanical prosthetic heart valves. FDA, USA; 2012. Available from:
www.fda.gov (Accessed
Feb, 2013).
- Price J, Hyynes M, Labinaz M, et al. Mechanical valve thrombosis with dabigatran. J Am Coll Cardiol 2012;60(17):1710–1.
- Stewart R, Astell H, Young L, White H. Thrombosis on a mechanical aortic valve whilst anti-coagulated with dabigatran.
Heart Lung Circ 2012;21:53–5.
- Chu J, Chen V, Dunton R. Thrombosis of a mechanical heart valve despite dabigatran. Ann Int Med 2012;157(4):304.
- Maegdefessel L, Linde T, Krapiec F, et al. In vitro comparison of dabigatran, unfractionated heparin, and low-molecular-weight
haparin in preventing thrombus formation on mechanical heart valves. Thromb Res 2010;126(3):e196–200.
- Stiles S. Dabigatran RE-ALIGN phase 2 mechanical-valve trial partly halted. The Heart 2012. Available from:
www.theheart.org (Accessed
Feb, 2013).
- Boehringer-Ingelheim. Boehringer Ingelheim discontinues Phase II trial in patients with artificial heart valves.
2012. Available from: www.boehringer-ingelheim.com (Accessed
Feb, 2013).
- Boehringer Ingelheim (NZ) Ltd. Dabigatran etexilate (Pradaxa) - Medicine data sheet. 2012. Available from:
www.medsafe.govt.nz (Accessed
Feb, 2013).
- New Zealand Formulary (NZF). NZF v8. NZF; 2013. Available from:
www.nzf.org.nz (Accessed
Feb, 2013).
- National Clinical Guideline Centre - Acute and Chronic Conditions. Venous thromboembolism: reducing the risk of
venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital). NICE, London;
2010. Available from: www.nice.org.uk (Accessed Feb, 2013).
- Kendoff D, Perka C, Fritsche H, et al. Oral thromboprophylaxis following total hip or knee replacement: Review and
multicentre experience with dabigatran etexilate. Open Orthop J 2011;5:395–9.
- Mahan C, Sryopoulos A. Improving prevention and treatment of venous thromboembolism: clinical trial results. J Med
Econ 2012;15(4):611–22.
- Friedman R, Dahl O, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after
hip or knee arthroplasty: A pooled anaylsis of three trials. Thromb Res 2010;126(3):175–82.
- Hart R, Diener H, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation
with warfarin or dabigatran. Stroke 2012;43(6):1511–7.
- Connolly S, Ezekowitz M, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Eng
J Med. 2009;361(12):1139–51.
- Connolly S, et al. Randomized comparison of the effects of two doses of dabigatran etexilate on clinical outcomes
over 4.3 years: results of the RELY-ABLE double-blind randomized trial. Clinical Science: Special Reports: Valvular
Heart Disease, PAD, Atrial Fibrillation: International Perspectives. USA: AHA; 2012.
- Hughes S. RELY-ABLE: Dabigatran looks good long term. The Heart 2012. Available from:
www.theheart.org (Accessed
Feb, 2013).
- Medsafe. Update on Pradaxa (Dabigatran etexilate). Medsafe prescriber update; 2012. Available from:
www.medsafe.govt.nz (Accessed
Feb, 2013).
- Liesenfeld K, Lehr T, Dansirikul C, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran
etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 2011;9(11):2168–75.
- Uchino K, Hernadez A. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority
randomized controlled trials. Arch Intern Med 2012;172:397–402.
- Granger C, Alexander J, McMurray J, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl
J Med 2011;365(11):981–92.
- Patel M, Mahaffey K, Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365(10):883–9.
- Rasmussen L, Larsen T, Graungaaed T, et al. Primary and secondary prevention with new oral anticolagulant drugs
for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ 2012;345:e7097.