 View / Download pdf version of this article
View / Download pdf version of this article
Key practice points:
- Non-pharmacological approaches are preferred for patients aged under 18 years with anxiety disorders or depression;
treatment should acknowledge the ongoing importance of family support, sleep, good nutrition and exercise
- Clinicians in primary care should consider consulting with a child and adolescent psychiatrist or paediatrician before
prescribing a psychoactive medicine to a patient aged under 18 years; these should only be prescribed if
symptoms are severe and/or other treatments have been ineffective and they are used alongside psychological
therapy
- There is evidence that selective serotonin reuptake inhibitors (SSRIs) may be effective for some young people with
severe or persistent anxiety or depression. These medicines are only approved for use in patients aged over 18 years
and their use in children and adolescents with depression or anxiety is almost always “off-label”.
- Fluoxetine offers the greatest benefit for young people with depression and is the only SSRI that should be initiated
in primary care without consulting with a child and adolescent psychiatrist. General practitioners may be
involved in continuing treatment with other SSRIs initiated in secondary care.
- The pharmacological treatment of mental health conditions in young people should be accompanied by increasing, rather
than decreasing, clinical contact. Frequent follow-up, e.g. weekly face-to-face or telephone contact, is
recommended for the first month of use.
 For previous articles in this series, see: “Addressing mental health and wellbeing in young people”,
BPJ 71 (Oct, 2015) and “Managing frequently encountered mental health problems in young people: non-pharmacological
strategies”, BPJ 72 (Dec, 2015)
For previous articles in this series, see: “Addressing mental health and wellbeing in young people”,
BPJ 71 (Oct, 2015) and “Managing frequently encountered mental health problems in young people: non-pharmacological
strategies”, BPJ 72 (Dec, 2015)
The use of psychoactive medicines in young people is increasing
The use of psychoactive medicines in people aged under 18 years has increased in New Zealand over the last five years
(Figure 1):1
- Dispensings of antidepressants to patients aged 10–17 years were 44% higher in 2014 than 2010. Fluoxetine accounted for 56% of SSRI dispensings in this age group in 2014.
- Antipsychotic dispensing to people aged 10–17 years increased by 48% in 2014 compared with 2010.
Quetiapine and risperidone are the most frequently dispensed antipsychotic medicines in this age group.
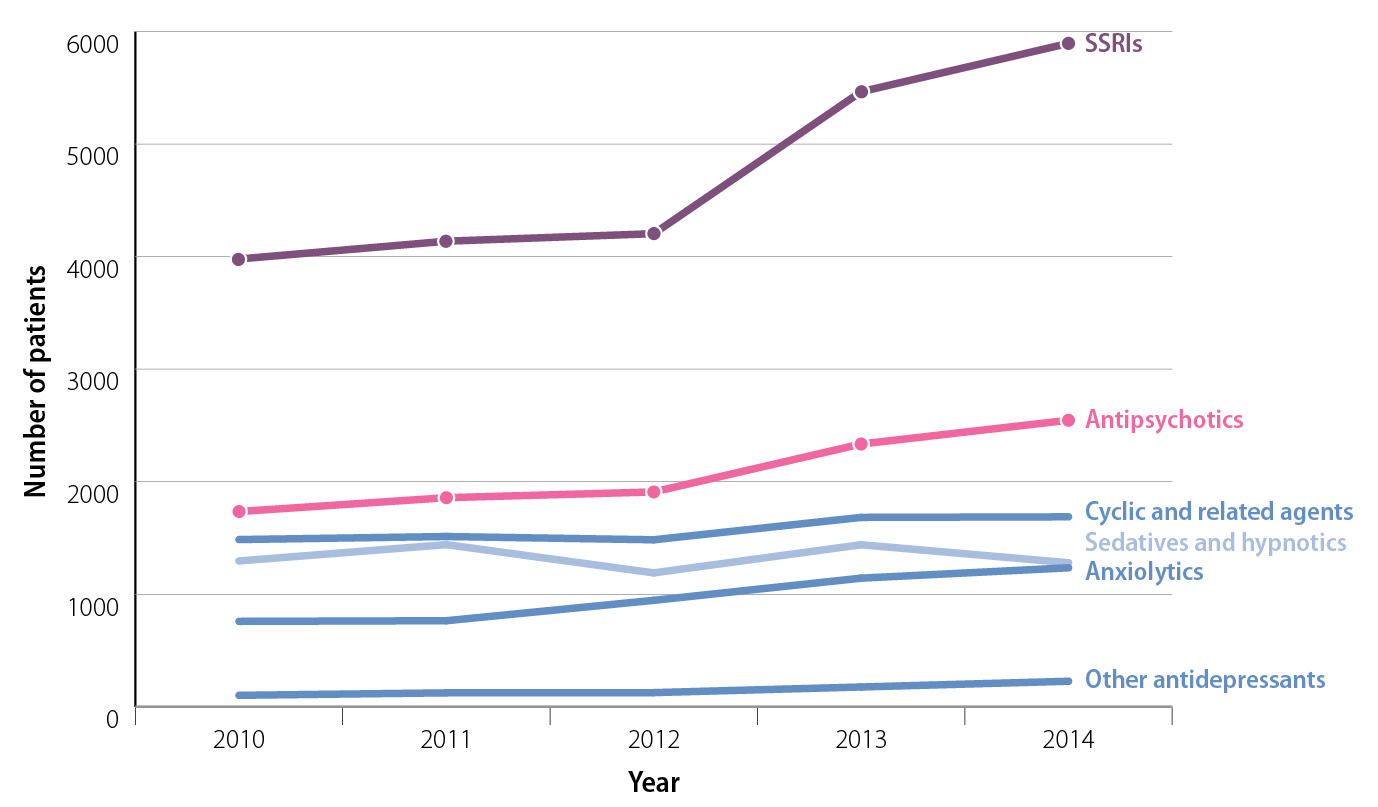
Figure 1:
New Zealand community dispensing for medicines used in the treatment of mental health conditions to people aged 10–17 years, from 2010 to 2014.1
 The individual medicines included in each group in Figure 1 are available from:
https://schedule.pharmac.govt.nz/ScheduleOnline.php?code=A22
The individual medicines included in each group in Figure 1 are available from:
https://schedule.pharmac.govt.nz/ScheduleOnline.php?code=A22
Why is dispensing of psychoactive medicines to young people increasing?
The most likely reason for the increase in dispensing of SSRIs to young people is a greater awareness of depression.
Clinicians may also be adopting a lower threshold for prescribing, as only small changes in the prevalence of mental health
conditions among young people have occurred.2,3
Guidance regarding the pharmacological
treatment of mental health conditions in young people has not changed substantially in the past five years. Furthermore,
the number of people aged 10–17 years in New Zealand actually decreased slightly from 2010 to 2014; therefore
population change cannot account for the increase.4
Medicines for mental health are often initiated in secondary care
Some classes of medicines used for the treatment of mental health conditions in young people are almost exclusively
initiated in secondary care. For example, antipsychotic medicines may be prescribed by child and adolescent psychiatrists
for the treatment of psychosis, bipolar disorder, anxiety and disruptive behaviours associated with autism, neurodevelopmental
disorders and conduct disorder in patients aged under 18 years. The majority of these medicines are not initiated
in primary care, but general practitioners may be required to continue treatment in consultation with the clinician
who initiated treatment (see: “Caring for patients aged under 18 years taking antipsychotic medicines”).
Most patients taking antidepressants will experience adverse effects
Most patients of any age who take an antidepressant experience at least one adverse effect. In short-term clinical trials
nausea is the most common adverse effect reported, occurring in approximately one in five patients, usually in the first
weeks of treatment and often resolving shortly after.5,6 Other adverse effects frequently reported include:7
- Agitation
- Changes in sexual function, such as erectile dysfunction and failure to orgasm
- Dizziness
- Drowsiness
- Dry mouth
- Headaches
- Psychological and emotional changes, such as blunted emotions or aggression
- Weight gain
Adverse events in young patients are more common and more severe
Younger patients are more likely to experience adverse effects while taking antidepressants than adults and these adverse
effects may be more severe.7,8 The evidence supporting SSRI use in young people has been questioned following
recent re-analyses of clinical trials which concluded that the harms of treatment appear to have been underestimated.8,9
Antidepressant treatment approximately doubles the rate of suicidal ideation and attempted suicide in children
and young people. However, the rates recorded in clinical trials are relatively low, e.g. 11 incidents per 1000 patients
taking placebo and 30 incidents per 1000 patients taking antidepressant medicines.8,10 This increase is
larger than that seen in adults taking antidepressants and involves suicidal thinking or suicide attempts: no studies
have reported an increase in the number of completed suicides for patients aged under 18 years using antidepressants.8,10
Antidepressants are not the first-line treatment for most young patients with depression or anxiety
For young people with depression or anxiety disorders, the use of counselling, psychological treatment, self-help and
online resources are the preferred first-line treatment options.11,12 Five or six out of every ten young
people with moderate to severe depression achieve remission with psychological treatment.13 In situations
where treatment with non-pharmacological approaches is unsuccessful, or difficult to access, other approaches may be necessary.
Second-line treatments can involve medicines or, where available, other non-pharmacological approaches, e.g. an escalation
from self-help to face-to-face counselling, or from counselling with a general practitioner to cognitive behavioural therapy
with a psychologist.
The evidence supporting antidepressant treatment in young people is limited
There are no medicines currently approved for the treatment of depression, generalised anxiety, social anxiety or panic
disorders in patients aged under 18 years in New Zealand.14 Although results from some clinical trials suggest
antidepressants are effective in patients aged under 18 years, worldwide few manufacturers have sought approval for their
use in young people.
For the treatment of depression fluoxetine is preferred in patients aged under 18 years due to its
superior risk-benefit profile. In patients aged under 18 years with depression the placebo effect, or natural rate of
remission, is larger than the effect produced by fluoxetine: two out of ten young people taking fluoxetine for depression
will experience natural remission or placebo effect, one will experience remission due to fluoxetine treatment and seven
will not experience a clinically significant improvement in symptoms.10 Antidepressant treatment is, however,
more likely to be effective for young patients with severe depression than those with mild to moderate depression.15
Fluoxetine has marketing authorisation in the United Kingdom for use in patients aged eight to 18 years, and is the
only antidepressant recommended for use in patients in this age group with severe or ongoing depression by the National
Institute for Health and Care Excellence (NICE).12 The Royal Australian and New Zealand College of Psychiatrists
also recommends that fluoxetine be considered, in addition to psychological treatment, in young people when depression
is moderate to severe or when psychotherapy has been ineffective.15
Paroxetine and venlafaxine should not be used in patients aged under 18 years; three placebo-controlled studies have
been conducted for each of these medicines which suggest they have limited efficacy.12 Tricyclic antidepressants
should not be used in patients aged under 18 years; it is unlikely they produce clinically significant benefits and they
are potentially toxic in overdose.12
For the treatment of anxiety disorders, e.g. panic disorder, social phobia or generalised anxiety disorder, there
is a wider range of antidepressants that may be appropriate in patients aged under 18 years, e.g. fluoxetine, sertraline,
venlafaxine.16,17 Generally, the use of these medicines is reserved for patients with severe or ongoing symptoms
who are likely to be referred to secondary care.
There is a strong placebo effect or natural rate of remission in young people with anxiety; response rates in placebo
groups in clinical trials range from 31–39%.16,17 In young patients with anxiety disorders SSRIs are almost
twice as effective as placebo.16,17
Balancing the risks and benefits of antidepressant treatment
It is unknown which patients with depressive symptoms will benefit prior to starting pharmacological treatment. This
makes it difficult to balance the risk versus the benefits of treatment, especially in young patients where the potential
harms may be greater. On one hand, the greatest risk associated with depression is suicide. On the other, antidepressants
increase the risk of suicidal ideation. Furthermore, suicide risk can be difficult to judge, is often highly variable
and can change rapidly in a young person.18
Similarly, the use of antidepressants may increase anxiety and agitation and patients with anxiety may experience a
worsening of symptoms.14
Consider how the patient’s condition affects their quality-of-life
For some young patients with mental health issues the concern may not be suicide risk but rather their long-term development.
In young people with persistent anxiety the principal concern could be the effect on their education and relationships.
It is also important to consider symptoms which are ongoing despite treatment, the concern of family members, relationships
or work commitments (see: “Case study: Lachie”).
Involve the patient’s family where appropriate
Involve the family or carers of young people, wherever possible, in treatment discussions for mental health conditions
if the patient consents; the observations of those closest to them are likely to be helpful, especially if a trial of
pharmacological treatment is initiated.
Situations where added caution is advised before initiating treatment
Situations where extra caution is advised before initiating antidepressant treatment for a young person in primary care
are shown in Table 1.
Table 1: Situations and reasons why trialling an antidepressant in primary care may not be appropriate.
| Scenario |
Factors for consideration |
Patient is a younger adolescent |
The evidence for treatment benefit is less certain in younger patients; the mean age of participants in clinical
trials for the treatment of depression in young patients with antidepressants ranges from 12 to 16 years10 |
Patient has a crisis which triggers an abrupt onset of depression, e.g. relationship ending |
It is important to differentiate between low mood arising from an event, e.g.grief or acute trauma, and long-term
changes in mood. The onset of action of fluoxetine is gradual; several weeks of treatment is required before a clinical
effect is experienced. Short-term pharmacological treatment is unlikely to be helpful for patients with an acute change
in mood caused by factors out of their control; counselling services and strong support is preferred in these situations. |
Patient is using alcohol or substances |
Alcohol and substance use are risk factors for suicide.18 The combination of antidepressant treatment
and alcohol or substance use may result in additional harm; young people with substance or alcohol
use problems should be referred to secondary care. |
Follow-up and monitoring is likely to be difficult |
The development of adverse effects may be difficult to detect in these situations, particularly if the patient
has limited family involvement, a history of non-attendance or is difficult to contact |
Patient has a pre-existing condition that increases the risk of adverse effects |
- Epilepsy; SSRIs antagonise the effect of antiepileptic medicines
- A history of mania
- Bleeding disorders or taking medicines which increase the risk of bleeding
- A high risk of QT prolongation*
|
*For information on the risk of QT prolongation with antipsychotic or antidepressant
medicines, see: www.medsafe.govt.nz/profs/PUArticles/DrugInducedQTProlongation.htm
Situations where it may be appropriate to initiate pharmacological treatment in primary care
While patients with mild to moderate depression or anxiety should be treated with non-pharmacological approaches before
medicines are trialled (see below), there may be situations where clinicians in primary care feel that more needs to be
done to help a young person.
The initiation of antidepressant treatment in primary care will often involve the input of a child and adolescent psychiatrist.
However, if the availability of psychological treatment is limited or if it is difficult for young people to attend therapy
sessions and online resources are insufficient, general practitioners may need to consider initiating a trial of pharmacological
treatment. In each case, clinicians will need to take an individualised approach taking into account the patient’s age,
history and circumstances, severity of symptoms, access to other treatments, wider family/whānau support and how they
have responded to non-pharmacological treatments.
Case study: Lachie
Lachie, aged 16 years, presents with his mother. He reports low mood, insomnia, and lethargy worsening over the preceding
two months. His mother is concerned: Lachie has missed school most days in the past fortnight. He was working through
the online SPARX (Smart, Positive, Active, Realistic, X-factor thoughts) programme with some benefit, but recently has
felt too low to continue.
Lachie has a strong family history of depression, with his older brother committing suicide at age 19 years. Lachie
denies any thoughts or plans of suicide or self harm at this time, but his mother is anxious.
Lachie is moderately depressed and risk factors include having a family member who committed suicide.18 On
balance it is reasonable to trial fluoxetine and the local adolescent psychiatrist agrees with this assessment and treatment
plan. Lachie is encouraged to continue with the SPARX programme and to make use of online or telephone support. He is
also referred to a child and adolescent psychologist to ensure that psychological therapy is continued.
The risks and benefits of fluoxetine are discussed with Lachie and his mother and its off-label use explained. Lachie
and his mother are warned about the potential for increased suicidality. Arrangements are made to see Lachie in one week
or sooner if required, and both Lachie and his mother are given the local psychiatric emergency phone number to call if
there is a sudden change in his condition.
 A directory of local mental health and support services is available
from: www.werrycentre.org.nz/service/locations?tid=168
A directory of local mental health and support services is available
from: www.werrycentre.org.nz/service/locations?tid=168
Parents, family members and friends of young people with mental health issues are likely to be affected by their change
in mood and behaviour. Given the family history of suicide in this case,
Lachie’s family members are likely to benefit from additional support.
Lachie’s mother is given the details of a local support service and encouraged to use online resources such
as Common Ground, available from: www.commonground.org.nz
Trialling pharmacological treatment in a young patient
Before initiating pharmacological treatment for depression or anxiety in a young person ensure that:
- Alternative treatment options, including available psychological therapies, and lifestyle advice regarding sleep,
nutrition and exercise have been discussed
- Consider whether a child and adolescent psychiatrist should be consulted, or an experienced colleague in primary
care if this is not possible
- The patient is not already taking an antidepressant prescribed by another clinician
- A plan is established for follow-up and monitoring in order to maintain contact with the patient
 For further information about healthy lifestyle advice which may improve a young person’s mood, see:
www.bpac.org.nz/BPJ/2015/December/mental-health.aspx#nutrition
For further information about healthy lifestyle advice which may improve a young person’s mood, see:
www.bpac.org.nz/BPJ/2015/December/mental-health.aspx#nutrition
Antidepressants are initiated as a trial
Whenever antidepressant medicines are initiated in patients aged under 18 years their use should be regarded as a trial.
Close contact and frequent follow-up are important in the weeks following initiation.
Advice for young people and their parents or carers should cover:12,19
- That the use of the medicine is “off-label” and what this means
- The need for psychological therapy to continue alongside pharmacological treatment
- The risks and benefits of treatment, particularly the increased risk of suicidal ideation or self-harm behaviours
- The contact details of a local emergency psychiatric service should the patient’s condition deteriorate or they develop
suicidal ideation or aggression
- That any benefits may take three to four weeks to occur; emphasise the importance of adherence to treatment
- That withdrawal symptoms may occur if a dose is missed or treatment is stopped suddenly
Remind patients to avoid using alcohol or illicit drugs as their disinhibiting effects may increase the risk of suicide.18 St.
John’s wort or supplements containing tryptophan should also be avoided as they may increase the risk of serotonin syndrome.20
 For further information on the use of medicines for unapproved indications, see:
www.bpac.org.nz/BPJ/2013/March/unapproved-medicines.aspx
For further information on the use of medicines for unapproved indications, see:
www.bpac.org.nz/BPJ/2013/March/unapproved-medicines.aspx
Dosing starts low with regular follow-up
Fluoxetine, initially 10 mg, daily, is suggested for the treatment of severe or ongoing depression in patients
aged under 18 years in primary care; this can be increased to 20 mg, daily, after one week if necessary; evidence is limited
regarding the efficacy of doses above 20 mg.12 Follow up should be weekly for the first month, then monthly.12
There is a wider range of antidepressants that may be effective for the treatment of anxiety disorders in young patients.17,
21 Clinicians in primary care should consult with a child and adolescent psychiatrist to determine whether initiating
one of these medicines is likely to be beneficial in a patient aged under 18 years with severe or ongoing anxiety.
Clinicians should review patients within one week of initiating antidepressants, and be alert for the development
of suicidal thinking or self-harm, particularly during the first month of use.12 Include the patient’s parents/carers
in the treatment plan, with the patient’s consent, as they may be well placed to judge treatment response and to monitor
the young person for adverse effects; consent from the patient may not be necessary if they are at risk of harming themselves.
If the patient shows an improvement with antidepressant treatment it may be continued with follow-up consultations
every one to two months.22 Treatment duration is typically at least six months.12 The decision to
continue treatment after a trial will depend on shared decision making with patients and parents/carers.
If patients develop adverse effects management strategies include:19
- Monitoring of symptoms, if they are mild and tolerable
- Discontinuing the medicine and increasing the use of psychological treatment
- Reducing the dose of the medicine for a period then increasing it if tolerated
- Switching to another medicine: if patients wish to trial another antidepressant referral to a child and adolescent
psychiatrist is recommended
If the patient develops an increase in suicidal ideation or self-harm, antidepressant medicines should be
discontinued. The patient should be urgently referred to child and adolescent mental health services;12,22 consultation
with a child and adolescent psychiatrist is also helpful to determine whether dose tapering is required to minimise any
adverse effects of withdrawal (see below). If a young patient develops an increase in suicidal ideation or self-harm while
using antidepressants more intensive psychological treatment or an alternative pharmacological approach may be necessary.
If there is no improvement with antidepressant treatment, or worsening of symptoms consultation with a child
and adolescent psychiatrist is recommended; dose adjustment, intensification of psychological approaches or a trial of
another antidepressant medicine may be necessary.
Fluoxetine withdrawal does not usually require dose tapering
In general, antidepressant medicines should be discontinued over the course of at least four weeks.6 However,
fluoxetine may be stopped in some patients without the need for dose tapering due to its long half-life and lower risk
of withdrawal symptoms.6 For young patients who have been taking a high dose of fluoxetine, i.e. 40 mg daily,
or a long course, e.g. months, dose tapering may be necessary.
The most common withdrawal symptoms for SSRIs include:14
- Gastro-intestinal disturbances
- Headache
- Dizziness
- Anxiety
- Paraesthesias, such as a sensation of an electric shock in neck, head or spine
- Tinnitus
- Fatigue
- Influenza-like symptoms
- Sweating
Clinicians should provide patients and family members or carers with a written action plan of who to contact if negative
changes in the young person’s behaviour, agitation or irritability occur during antidepressant withdrawal.22
Caring for patients aged under 18 years taking antipsychotic medicines
Young people treated with antipsychotics are at an increased risk of metabolic adverse effects, in particular rapid
weight gain, compared with adults using the same medicines.23 Weight gain in young people with mental health
conditions may have a negative effect on interactions with peers and their quality of life. Young people taking antipsychotics
are also two to three times more likely to develop type 2 diabetes than their peers.24
Before initiating antipsychotic medicines in young people, the following examinations are recommended:25
- Lipid levels: triglycerides, total cholesterol and total:HDL cholesterol ratio
- Fasting glucose
- Blood pressure
- BMI and waist circumference
- Baseline ECG for patients with risk factors for QT prolongation*
Where appropriate, repeat these measurements at suggested intervals of:25
- Six weeks after starting antipsychotic medicines: BMI and waist circumference
- Then 12 weeks after initiation: all measures
- Then annually thereafter: all measures
Additional tests may be needed depending on the patient’s symptoms and signs, e.g. prolactin levels may be assessed
in young people with symptoms suggestive of hyperprolactinaemia: e.g. changes in sexual function in teenagers who are
sexually active, menstruation changes or the production of breast milk.25
Ask about or examine the patient for the development of:26,27
- Extrapyramidal effects, e.g. tardive dyskinesia
- Drowsiness
- Nocturnal enuresis (bed wetting)
Treatment should be discontinued if tardive dyskinesia develops. For the management of other extrapyramidal adverse
effects, options include dose reduction, discontinuing medicines, or the use of anticholinergic medicines to reduce extrapyramidal
symptoms. If a patient develops any of these symptoms consultation with the psychiatrist who is managing their care is
recommended.28
* See:www.medsafe.govt.nz/profs/PUArticles/DrugInducedQTProlongation.htm
Acknowledgement
Thank you to Dr Sue Bagshaw, Senior Medical Officer Youth Health, Director of the Collaborative for Research and Training in Youth
Health and Development Trust, Senior Lecturer, Paediatrics, University of Otago, Christchurch, and Dr
Hiran Thabrew, Child and
Adolescent Psychiatrist and Paediatrician, Deputy Director of the Werry Centre, Senior Lecturer, Psychological Medicine,
University of Auckland for expert review of this article
References
- Ministry of Health. Pharmaceutical Claims Collection. 2015.
- Fleming TM, Clark T, Denny S, et al. Stability and change in the mental health of New Zealand secondary school students
2007-2012: results from the national adolescent health surveys. Aust N Z J Psychiatry 2014;48:472–80. http://dx.doi.org/10.1177/0004867413514489
- Ministry of Health. Tier 1 statistics 2014/15: New Zealand Health Survey. Ministry of Health NZ. 2015. Available
from: www.health.govt.nz/publication/tier-1-statistics-2014-15-new-zealand-health-survey (Accessed
Mar, 2016).
- Statistics New Zealand. Infoshare, Population estimates - DPE, estimated resident population by age and sex, 1991+,
annual-Jun. 2015. Available from: www.stats.govt.nz/infoshare/default.aspx (Accessed
Mar, 2016).
- Greist J, McNamara RK, Mallinckrodt CH, et al. Incidence and duration of antidepressant-induced nausea: duloxetine
compared with paroxetine and fluoxetine. Clin Ther 2004;26:1446–55. http://dx.doi.org/10.1016/j.clinthera.2004.09.010
- National Collaborating Centre for Mental Health (UK). Depression: the treatment and management of depression in adults
(updated edition). Leicester (UK): British Psychological Society 2010. Available from: www.ncbi.nlm.nih.gov/books/NBK63748/ (Accessed
Mar, 2016).
- Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while
taking antidepressants. Psychiatry Res 2014;216:67–73. http://dx.doi.org/10.1016/j.psychres.2014.01.042
- Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review
and meta-analyses based on clinical study reports. BMJ 2016;352:i65. http://dx.doi.org/10.1136/bmj.i65
- Le Noury J, Nardo JM, Healy D, et al. Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment
of major depression in adolescence. BMJ 2015;351:h4320.http://dx.doi.org/10.1136/bmj.h4320
- Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and
adolescents. Cochrane Database Syst Rev 2012;11:CD004851. http://dx.doi.org/10.1002/14651858.CD004851.pub3
- National Institute for Health Care Excellence (NICE). Social anxiety disorder: recognition, assessment and treatment.
London: NICE 2013. Available from: www.nice.org.uk/guidance/cg159 (Accessed
Mar, 2016).
- National Institute for Health and Care Excellence (NICE). Depression in children and young people: identification
and management. London: NICE 2005. Available from: www.nice.org.uk/guidance/cg28 (Accessed
Mar, 2016).
- Cox GR, Callahan P, Churchill R, et al. Psychological therapies versus antidepressant medication, alone and in combination
for depression in children and adolescents. Cochrane Database Syst Rev 2014;11:CD008324. http://dx.doi.org/10.1002/14651858.CD008324.pub3
- New Zealand Formulary for Children. NZFC v44. 2016. Available from: www.nzfchildren.org.nz/ (Accessed
Mar, 2016).
- Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice
guidelines for mood disorders. Aust N Z J Psychiatry 2015;49:1087–206. http://dx.doi.org/10.1177/0004867415617657
- Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts
in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007;297:1683–96. http://dx.doi.org/10.1001/jama.297.15.1683
- Ipser JC, Stein DJ, Hawkridge S, et al. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane
Database Syst Rev 2009;3:CD005170. http://dx.doi.org/10.1002/14651858.CD005170.pub2
- New Zealand Guidelines Group. Assessment and management of people at risk of suicide. Ministry of Health NZ 2003.
Available from: www.health.govt.nz/publication/assessment-and-management-people-risk-suicide (Accessed
Mar, 2016).
- National Institute for Health and Care Excellence (NICE). Generalised anxiety disorder and panic disorder in adults.
London: NICE 2011. Available from: www.nice.org.uk/guidance/cg113 (Accessed
Mar, 2016).
- Ables AZ, Nagubilli R. Prevention, recognition, and management of serotonin syndrome. Am Fam Physician 2010;81:1139–42.
- Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders:
a systematic review and meta-analysis. Depress Anxiety 2015;32:149–57. http://dx.doi.org/10.1002/da.22329
- New Zealand Guidelines Group (NZGG). Identification of common mental disorders and management of depression in primary
care. Wellington: NZGG 2008. Available from: www.health.govt.nz/system/files/documents/publications/depression_guideline.pdf (Accessed
Mar, 2016).
- De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position
statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes
(EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009;24:412–24. http://dx.doi.org/10.1016/j.eurpsy.2009.01.005
- Galling B, Roldán A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: A systematic
review and meta-analysis. JAMA Psychiatry 2016;73:247–59. http://dx.doi.org/10.1001/jamapsychiatry.2015.2923
- De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia:
systematic evaluation. Br J Psychiatry 2011;199:99–105. http://dx.doi.org/10.1192/bjp.bp.110.084665
- Cohen D, Bonnot O, Bodeau N, et al. Adverse effects of second-generation antipsychotics in children and adolescents:
a Bayesian meta-analysis. J Clin Psychopharmacol 2012;32:309–16. http://dx.doi.org/10.1097/JCP.0b013e3182549259
- Barnes TRE, Drake MJ, Paton C. Nocturnal enuresis with antipsychotic medication. Br J Psychiatry 2012;200:7–9. http://dx.doi.org/10.1192/bjp.bp.111.095737
- Pringsheim T, Doja A, Belanger S, et al. Treatment recommendations for extrapyramidal side effects associated with
second-generation antipsychotic use in children and youth. Paediatr Child Health 2011;16:590–8.